In pharmaceutical testing, disintegration and dissolution play crucial roles in ensuring effective drug development. These processes determine how medications break down and dissolve, impacting bioavailability and patient safety. Let’s dive into their key differences and importance.
Contents
The Key to Effective Drug Development
In pharmaceutical testing, terms like disintegration and dissolution are vital for drug development. These processes ensure medications work effectively. Let’s explore their differences, importance, and applications in scientific analysis for better healthcare solutions.
What Is Disintegration?
Breaking Down the Basics
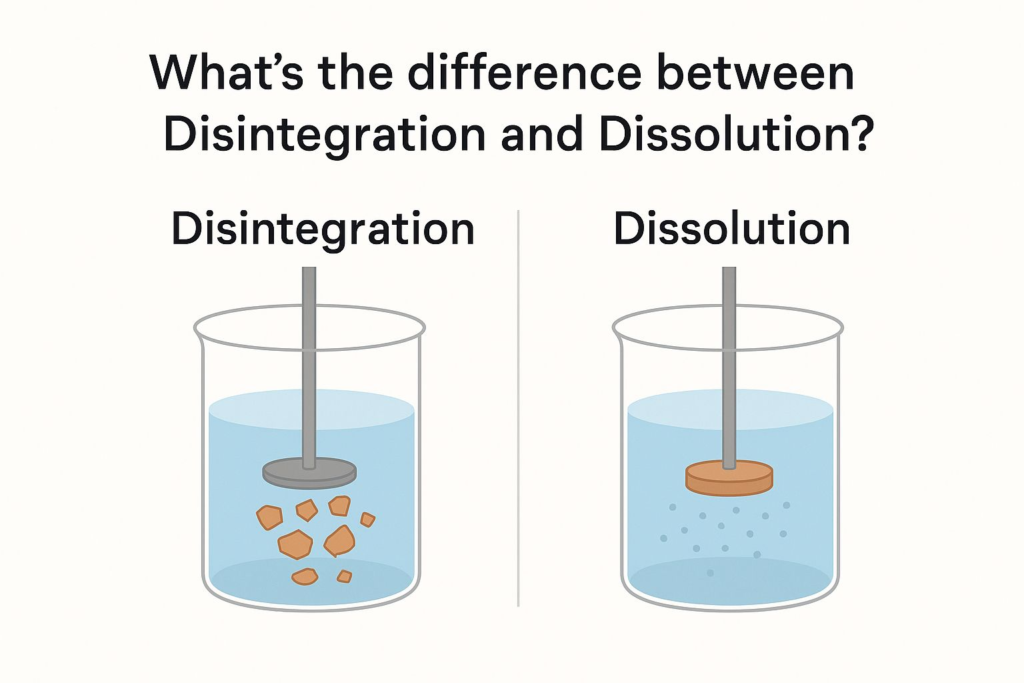
Disintegration refers to a solid dosage form, like a tablet, breaking into smaller fragments in a liquid medium. This physical process, often tested in labs, ensures the drug can release its active ingredients for absorption during treatment.
What Is Dissolution?
The Science of Solubility
Dissolution is the process where these fragments dissolve into a solution, making the drug available for absorption. It’s a critical step in drug development, as it measures how quickly a drug becomes bioavailable in the body.
Disintegration vs. Dissolution: A Side-by-Side Comparison
Here’s a clear comparison to highlight their roles in pharmaceutical testing:
| Aspect | Disintegration | Dissolution |
|---|---|---|
| Definition | Tablet breaks into smaller pieces | Drug dissolves into a solution |
| Focus | Physical breakdown | Chemical solubility |
| Testing Purpose | Ensures drug release | Measures absorption rate |
| Medium | Usually water or simulated fluid | Simulated gastric or intestinal fluid |
Why Are These Processes Important?
Impact on Drug Development
- Quality Control: Ensures consistency in drug manufacturing.
- Bioavailability: Determines how much drug reaches the bloodstream.
- Regulatory Compliance: Meets standards set by agencies like the FDA.
- Patient Safety: Guarantees effective and safe medication delivery.
Applications in Scientific Analysis
Enhancing Pharmaceutical Research
Disintegration and dissolution testing are crucial in scientific analysis. They help researchers optimize formulations, improve drug efficacy, and reduce development costs. These tests are standard in labs to meet global pharmaceutical standards and ensure patient outcomes.
Conclusion: Driving Innovation in Healthcare
Understanding disintegration and dissolution is essential for advancing drug development. These processes, integral to pharmaceutical testing, ensure medications are safe, effective, and reliable. For more insights, check resources from USP to stay updated.

More questions and answers send
Ok we Will publish more articles on it