Explore pharma jobs in Hyderabad at Dr. Reddy’s Career Expo. Walk-in interview for manufacturing and QA/QC jobs with 3-10 years of experience. Kickstart your pharmaceutical careers in India—attend on Nov 9!
Contents
- 1 About the Company
- 2 Job Details
- 3 Job Description
- 3.1 Team Member – Packing
- 3.2 TM Engineering – Drug Product / Sterile/Injectable (I&A)
- 3.3 TM Engineering – Drug Substance Mechanical
- 3.4 Team Member – Drug Substance Upstream / Downstream
- 3.5 Team Member MSAT – Upstream / Downstream
- 3.6 Development Quality Assurance
- 3.7 Quality Control – Bioassay
- 3.8 Drug Substance – Quality Assurance
- 3.9 Quality Control – RM/PM
- 3.10 ASAT – Biosimilar
- 3.11 Quality Control – Lab Support
- 3.12 Quality Control Associate Analytical
- 3.13 Quality Control – Stability
- 4 Skills/Qualifications
- 5 Key Responsibilities
- 6 Benefits/Perks
- 7 How to Apply
- 8 Walk-in Interview Details
- 9 Why You Should Join
- 10 FAQs
About the Company
Dr. Reddy’s Laboratories Ltd., established in 1984, is a premier global pharmaceutical powerhouse headquartered in Hyderabad, India. Renowned for its innovation in generics, biosimilars, and differentiated formulations, the company prioritizes patient-centric solutions and regulatory excellence.
With USFDA, EMA, and WHO approvals, Dr. Reddy’s operates in over 100 countries, driving growth through R&D investments exceeding $100 million annually. Its commitment to cGMP compliance and sustainable practices makes it a top choice for pharmaceutical careers in India, fostering talent in cutting-edge biopharma environments.
Job Details
- Company Name: Dr. Reddy’s Laboratories Ltd.
- Experience: 3 to 10 Years
- Qualification: Diploma/B.Tech/B.Sc/M.Sc/B.Pharm/B.Tech or Masters in Biotechnology/Microbiology/Biochemistry
- Location: Bachupally / Shamirpet, Hyderabad
- Work Type: On-site
Job Description
Dr. Reddy’s is hosting a dynamic Career Expo to recruit passionate professionals for manufacturing and quality roles, emphasizing innovation in drug substance, drug product, and biologics.
These positions demand expertise in GMP compliance and advanced biopharma processes, offering a gateway to impactful pharma jobs. With a focus on upstream/downstream operations and analytical excellence, join a team accelerating “Good Health Can’t Wait.”
Team Member – Packing
- Department: Manufacturing
- Role: Medical device packaging and visual inspection with cGMP alignment
- Experience: 3 to 10 Years
- Education/Qualification: Diploma/B.Tech/B.Sc/M.Sc/B.Pharm
TM Engineering – Drug Product / Sterile/Injectable (I&A)
- Department: Manufacturing
- Role: Instrumentation & Automation for vial filling, washing, and tunnel systems
- Experience: 3 to 10 Years
- Education/Qualification: Diploma/B.Tech/B.Sc/M.Sc/B.Pharm
TM Engineering – Drug Substance Mechanical
- Department: Manufacturing
- Role: Automation platforms including ABB800xA, Siemens, Allen Bradley PLCs, SCADA, and biopharma equipment like bioreactors and TFF
- Experience: 3 to 10 Years
- Education/Qualification: Diploma/B.Tech/B.Sc/M.Sc/B.Pharm
Team Member – Drug Substance Upstream / Downstream
- Department: Manufacturing
- Role: Execute upstream/downstream unit operations with GMP documentation and compliance
- Experience: 3 to 10 Years
- Education/Qualification: Diploma/B.Tech/B.Sc/M.Sc/B.Pharm
Team Member MSAT – Upstream / Downstream
- Department: Manufacturing
- Role: Specialist in mammalian cell culture, perfusion, scale-up, single-use tech, bioreactor characterization, chromatography, ultrafiltration, and virus filtration
- Experience: 3 to 10 Years
- Education/Qualification: Diploma/B.Tech/B.Sc/M.Sc/B.Pharm
Development Quality Assurance
- Department: Quality
- Role: QA in drug substance, drug product, and analytical development
- Experience: 3 to 10 Years
- Education/Qualification: B.Tech or Masters in Biotechnology/Microbiology/Biochemistry
Quality Control – Bioassay
- Department: Quality
- Role: Cell-based bioassays, SPR, and FACS techniques
- Experience: 3 to 10 Years
- Education/Qualification: B.Tech or Masters in Biotechnology/Microbiology/Biochemistry
Drug Substance – Quality Assurance
- Department: Quality
- Role: In-process QA for drug substance manufacturing, upstream, and downstream
- Experience: 3 to 10 Years
- Education/Qualification: B.Tech or Masters in Biotechnology/Microbiology/Biochemistry
Quality Control – RM/PM
- Department: Quality
- Role: Material analytics lab with GLP adherence and safety practices; sample analysis via analytical techniques
- Experience: 3 to 10 Years
- Education/Qualification: B.Tech or Masters in Biotechnology/Microbiology/Biochemistry
ASAT – Biosimilar
- Department: Quality
- Role: Analytical method transfers, validation, real-time investigations, annual performance, and query resolution
- Experience: 3 to 10 Years
- Education/Qualification: B.Tech or Masters in Biotechnology/Microbiology/Biochemistry
Quality Control – Lab Support
- Department: Quality
- Role: Equipment qualification, calibration, periodic GxP reviews per GAMP5 and 21CFR
- Experience: 3 to 10 Years
- Education/Qualification: B.Tech or Masters in Biotechnology/Microbiology/Biochemistry
Quality Control Associate Analytical
- Department: Quality
- Role: Lab analysis for biosimilars/biologics using RP-HPLC, Glycan, SEC, IEX, PepMap
- Experience: 3 to 10 Years
- Education/Qualification: B.Tech or Masters in Biotechnology/Microbiology/Biochemistry
Quality Control – Stability
- Department: Quality
- Role: Stability study protocols/reports, batch planning, chamber maintenance per cGMP
- Experience: 3 to 10 Years
- Education/Qualification: B.Tech or Masters in Biotechnology/Microbiology/Biochemistry
Skills/Qualifications
- Expertise in cGMP, GLP, GAMP5, and 21CFR compliance
- Proficiency in biopharma equipment like bioreactors, TFF, ATF, and automation platforms (ABB, Siemens, Allen Bradley)
- Hands-on experience in upstream/downstream operations, cell culture, chromatography, and ultrafiltration
- Strong analytical skills in HPLC, bioassays, SPR, FACS, and stability studies
- Knowledge of documentation, strategic planning, and safety practices
- Preferred regulatory exposure including ICH guidelines and QMS
Key Responsibilities
- Execute unit operations and inspections
- Validate methods and troubleshoot processes
- Maintain equipment and perform calibrations
- Document compliance and investigate deviations
- Analyze samples using advanced techniques
- Support scale-up and stability studies
- Ensure GMP and regulatory adherence
Benefits/Perks
- Accelerated career growth in biosimilars and generics
- Extensive training in cutting-edge biopharma tech
- Competitive salary with performance bonuses
- Inclusive work culture promoting innovation
- Global exposure via international collaborations
How to Apply
Attend the Career Expo to apply directly—scan the QR code at the venue for instant registration. Bring your resume and qualifications. For additional pharma jobs, visit Pharma Recruiter. Seize this chance for QA jobs and manufacturing roles in pharmaceutical careers in India—register today!

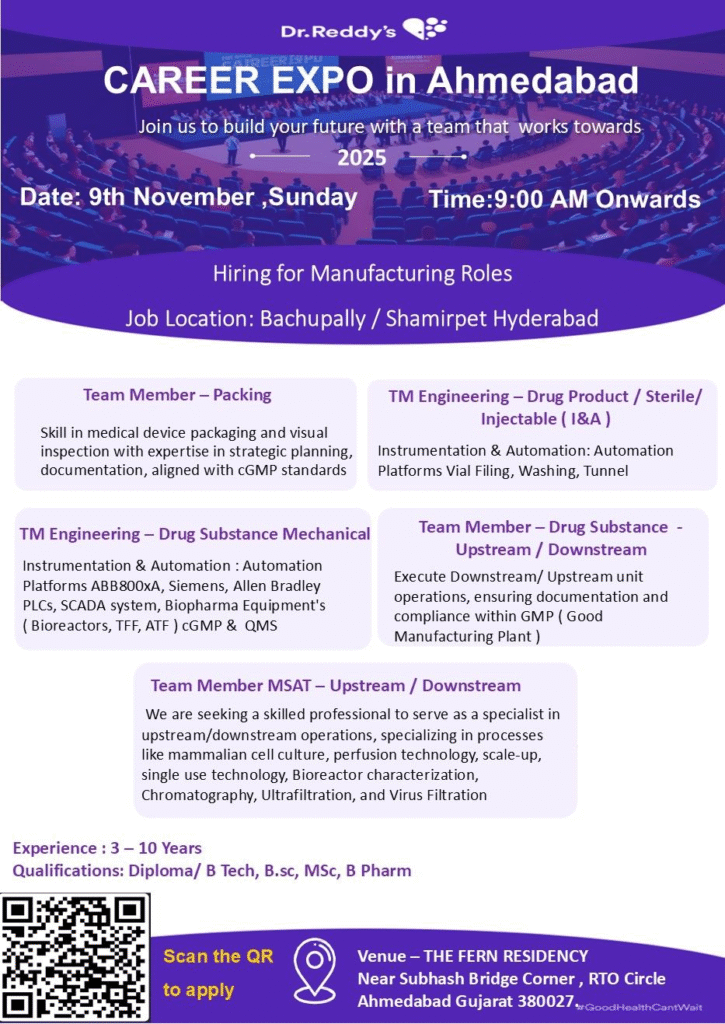
Walk-in Interview Details
- Date: 9th November 2025 (Sunday)
- Time: 9:00 AM Onwards
- Venue: The Fern Residency, Near Subhash Bridge Corner, RTO Circle, Ahmedabad, Gujarat 380027
- Contact/Email: Scan QR at venue or visit Dr. Reddy’s careers page
Why You Should Join
Dr. Reddy’s cultivates a vibrant culture of recognition, where your expertise in QC jobs and production fuels breakthroughs in affordable healthcare. Benefit from unmatched career stability in a $3B+ revenue giant, with personalized growth paths and leadership programs. Immerse in a compliant yet innovative ecosystem, advancing biosimilars and sterile injectables for global impact.
FAQs
What eligibility is required for these pharma jobs?
3-10 years experience with Diploma/B.Tech/B.Sc/M.Sc/B.Pharm or Masters in Biotech/Micro/Biochem; GMP knowledge essential.
How does the application process work at the expo?
Scan QR code on-site for quick submission; bring resume for immediate interviews in manufacturing and quality roles.
What to expect at the walk-in interview?
From 9 AM, engage with recruiters for QA jobs—discuss skills in automation and bioassays for Hyderabad positions.
What growth opportunities exist here?
Fast-track promotions, global R&D exposure, and training in biopharma innovations for sustained pharmaceutical careers.


