Discover Emcure’s walk-in interview for pharma jobs in Mehsana. Explore QA jobs, QC jobs, production jobs, and pharmaceutical careers in India at a global leader in oncology and injectables.
Contents
About the Company
Emcure Pharmaceuticals Limited, founded in 1981 and headquartered in Pune, India, is a leading multinational pharmaceutical company specializing in the development, manufacturing, and marketing of innovative therapies.
With a global footprint across India, Europe, Canada, Russia, Brazil, and beyond, Emcure excels in gynecology, cardiovascular, oncology, HIV antivirals, and blood-related disorders.
Through its subsidiary Gennova Biopharmaceuticals, it pioneers biotech solutions for life-threatening diseases, ensuring regulatory compliance and high-quality standards in OSD and injectable facilities to deliver affordable healthcare worldwide.
Job Details
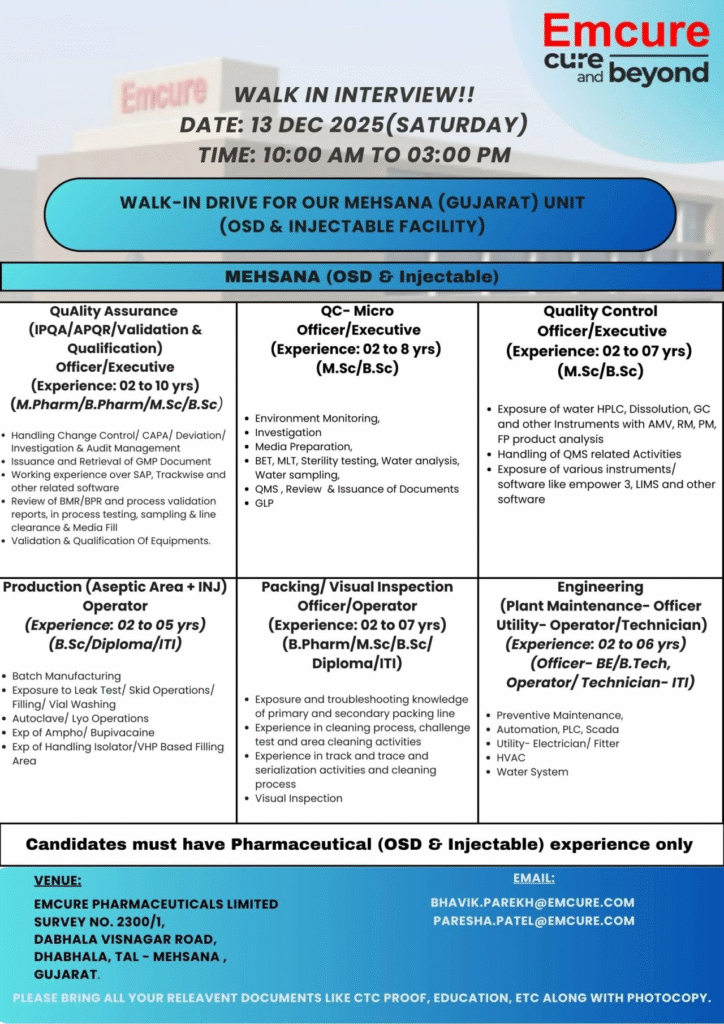
- Company Name: Emcure Pharmaceuticals Limited
- Experience: Varies by role (2 to 10 years)
- Qualification: M.Pharm/B.Pharm/M.Sc/B.Sc/Diploma/ITI/BE/B.Tech
- Location: Mehsana, Gujarat (OSD & Injectable Facility)
- Work Type: On-site
Job Description
Emcure Pharmaceuticals is hosting a walk-in drive for its state-of-the-art OSD and injectable unit in Mehsana, seeking experienced talent in quality, production, and engineering.
These pharma jobs prioritize GMP compliance and hands-on expertise in regulated environments, supporting the company’s global pipeline in oncology and antivirals. Multiple roles offer pathways for pharmaceutical careers in India with immediate impact.
Quality Assurance (IPQA/APQR/Validation & Qualification)
- Department: Quality Assurance
- Experience: 2 to 10 years
- Education/Qualification: M.Pharm/B.Pharm/M.Sc/B.Sc
QC-Micro Officer/Executive
- Department: Quality Control – Microbiology
- Experience: 2 to 8 years
- Education/Qualification: M.Sc/B.Sc
Quality Control Officer/Executive
- Department: Quality Control
- Experience: 2 to 07 years
- Education/Qualification: M.Sc/B.Sc
Production (Aseptic Area + INJ) Operator
- Department: Production (Aseptic Area + Injectables)
- Experience: 2 to 5 years
- Education/Qualification: B.Sc/Diploma/ITI
Packing/Visual Inspection Officer/Operator
- Department: Packing/Visual Inspection
- Experience: 2 to 7 years
- Education/Qualification: B.Pharm/M.Sc/B.Sc/Diploma/ITI
Engineering (Plant Maintenance – Officer Utility – Operator/Technician)
- Department: Engineering (Plant Maintenance & Utilities)
- Experience: 2 to 6 years
- Education/Qualification: Officer – BE/B.Tech; Operator/Technician – ITI
Skills/Qualifications
- Expertise in GMP documentation, SAP, Trackwise, and related software
- Proficiency in HPLC, GC, Dissolution, BET, MLT, Sterility testing
- Hands-on knowledge of change control, CAPA, deviations, and audits
- Experience in aseptic operations, leak testing, autoclave, and lyophilization
- Familiarity with isolator/VHP-based filling, HVAC, PLC, and SCADA systems
- Strong troubleshooting for packing lines, serialization, and media fill
- Relevant pharma experience in OSD and injectable facilities only
Key Responsibilities
- Manage change controls, CAPA, deviations, and audit preparations
- Conduct environmental monitoring, media prep, and water analysis
- Perform HPLC/GC assays, RM/PM/FP analysis, and QMS activities
- Execute batch manufacturing, filling, vial washing, and leak tests
- Oversee visual inspections, packing line troubleshooting, and serialization
- Handle preventive maintenance, utility operations, and equipment validation
Benefits/Perks
- Career growth in a global pharma innovator with biotech focus
- Exposure to regulated OSD and injectable manufacturing
- Competitive salary and performance incentives
- Collaborative culture emphasizing compliance and innovation
- Opportunities for skill development in advanced pharma technologies
How to Apply
Attend the walk-in interview on 13 December 2025 with your updated resume, CTC proof, educational certificates, experience letters, and photocopies. Only candidates with OSD and injectable pharma experience qualify. For queries, email bhavik.parekh@emcure.com or paresh.patel@emcure.com.
Discover more pharma jobs via Pharma Recruiter. Act fast—walk in tomorrow and elevate your pharmaceutical careers in India!
Walk-in Interview Details
- Date: 13 December 2025 (Saturday)
- Time: 10:00 AM to 03:00 PM
- Venue: Emcure Pharmaceuticals Limited, Survey No. 2300/1, Dabhala Visnagar Road, Dabhala, Tal – Mehsana, Gujarat
- Contact/Email: bhavik.parekh@emcure.com | paresh.patel@emcure.com
Why You Should Join
Emcure Pharmaceuticals embodies a culture of relentless innovation and patient-centric excellence, certified for its global standards in oncology and HIV therapies. Employees benefit from long-term career stability within a multinational powerhouse, with abundant learning opportunities in cutting-edge injectable and biotech projects.
Immerse yourself in a compliance-rich environment that rewards expertise, fostering collaborative growth and meaningful contributions to affordable medicines for diverse therapeutic needs worldwide.
FAQs
What experience is required for QA jobs at Emcure’s Mehsana unit?
2-10 years in IPQA, validation, or qualification, with pharma OSD/injectable background; M.Pharm/B.Pharm/M.Sc/B.Sc preferred.
How do I prepare for the walk-in interview for production jobs?
Bring resume, CTC proof, and documents; focus on aseptic operations, leak testing, and GMP knowledge for on-site screening.
Where is the venue for QC jobs walk-in, and when is it?
At Emcure’s Mehsana facility on 13 Dec 2025, 10 AM-3 PM; email queries to HR for guidance.
What growth opportunities exist in pharmaceutical careers at Emcure?
Advance in global R&D, gain biotech exposure via Gennova, and thrive in a dynamic, ethics-driven culture with international reach.


