Emcure Pharmaceuticals Ltd, a leading Indian multinational, is hosting a Walk-In Interview on May 9, 2025, at its USFDA-approved oral solid dosage (OSD) plant in Kadu, Surendranagar, Gujarat. Founded in 1983, Emcure produces 350+ formulations for oncology, cardiology, and diabetes, exporting to 70+ countries with ₹8,000 Crore revenue.
Rated 3.7/5 for work culture on AmbitionBox (1.9k reviews), it scores 3.5/5 for work-life balance due to shift demands. This recruitment targets Production, Engineering, Quality Assurance (QA), Quality Control (QC), Warehouse, and IT-Computer System Validation (CSV) roles, offering ₹1.8–7 Lakhs per annum (Indeed). ITI freshers welcome! Apply by May 9, 2025!
Contents
- 1 Walk-In Interview Details
- 2 Why Join Emcure?
- 3 Job Positions
- 3.1 1. Production (OSD) – Operator / Technician / Officer
- 3.2 2. Engineering – Technician / Operator / Officer
- 3.3 3. Quality Assurance (QA) – Sr. Officer / Officer / Jr. Officer
- 3.4 4. Quality Control (QC) – Officer / Executive
- 3.5 5. Warehouse – Officer / Executive
- 3.6 6. IT-Computer System Validation (CSV) – Officer / Executive
- 3.7 Eligibility Criteria
- 4 Why These Roles?
- 5 How to Prepare for the Walk-In
- 6 Why Surendranagar, Gujarat?
- 7 Contact Information
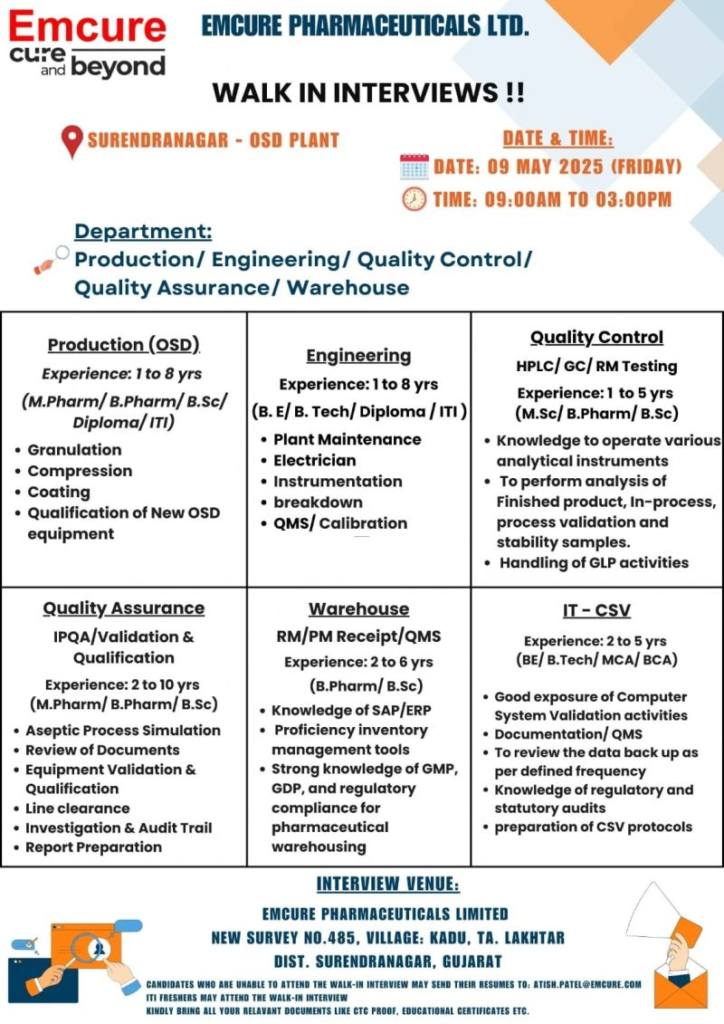
Walk-In Interview Details
- Date: Friday, May 9, 2025
- Time: 9:00 AM – 3:00 PM
- Venue: Emcure Pharmaceuticals Ltd, New Survey No. 485, Village Kadu, Taluka Lakhtar, Surendranagar, Gujarat 382775
- Application Method: Attend walk-in with documents or email CV to atish.patel@emcure.com, subject: “[Department] – Surendranagar OSD.”
- Contact: atish.patel@emcure.com, +91-2717-610-000
- Website: www.emcure.com
Notes:
- Fraud Alert: Emcure does not charge fees. Verify via www.emcure.com.
- ITI freshers (2024–2025) eligible for Production/Engineering.
Why Join Emcure?
Emcure’s Surendranagar OSD plant, a cGMP-compliant facility, produces tablets and capsules for global markets, with no USFDA 483s in 2024. With 8,600+ employees, Emcure supports skill development (3.8/5) but faces feedback on management (3.6/5). Join India’s $24.4 billion pharma export market, growing at 10% CAGR.
Job Positions
Emcure is recruiting for its Surendranagar OSD plant, emphasizing Cure Beyond.
1. Production (OSD) – Operator / Technician / Officer
- Sections: Granulation, Compression, Coating
- Qualification: M.Pharm, B.Pharm, B.Sc, Diploma, ITI
- Experience: 1–8 years in OSD; ITI freshers (2024–2025) eligible
- Vacancies: ~10–15 expected
- Responsibilities:
- Operate granulation (RMG, FBD), compression (Fette), and coating equipment.
- Qualify new OSD equipment per IQ/OQ/PQ protocols.
- Maintain BMR, BPR, and cGMP compliance.
- Skills: Knowledge of FBD, HATA, CVC packing; QMS handling.
- Salary: ₹1.8–5 Lakhs per annum (Indeed).
2. Engineering – Technician / Operator / Officer
- Sections: Plant Maintenance, Electrician, Instrumentation, Breakdown, QMS/Calibration
- Qualification: B.E, B.Tech, Diploma, ITI (Mechanical, Electrical, Instrumentation)
- Experience: 1–8 years; ITI freshers (2024–2025) eligible
- Vacancies: ~5–10 expected
- Responsibilities:
- Perform maintenance on FBD, RMG, and compression machines.
- Conduct calibration and QMS per cGMP.
- Troubleshoot PLC-based systems.
- Skills: Expertise in PLC, SCADA, P&ID; breakdown analysis.
- Salary: ₹2–6 Lakhs per annum (Indeed).
3. Quality Assurance (QA) – Sr. Officer / Officer / Jr. Officer
- Sections: IPQA, Validation & Qualification, Aseptic Process Simulation, Documentation, Audits
- Qualification: M.Pharm, B.Pharm, B.Sc
- Experience: 2–10 years in OSD QA
- Vacancies: ~5–8 expected
- Responsibilities:
- Conduct IPQA, line clearance, and aseptic process simulation.
- Review BMR, BPR, CAPA, and audit trails.
- Validate equipment per USFDA/MHRA standards.
- Skills: Proficiency in QMS, SAP, TrackWise; investigation skills.
- Salary: ₹3–7 Lakhs per annum (AmbitionBox).
4. Quality Control (QC) – Officer / Executive
- Sections: HPLC, GC, RM Testing, GLP Activities
- Qualification: M.Sc, B.Pharm, B.Sc
- Experience: 1–5 years in OSD QC
- Vacancies: ~5–10 expected
- Responsibilities:
- Analyze samples using HPLC, GC, and dissolution testers.
- Perform GLP activities and maintain QMS records.
- Support process validation and audits.
- Skills: Expertise in Empower 3, LIMS, HPLC; analytical accuracy.
- Salary: ₹2.5–5.5 Lakhs per annum (Indeed).
5. Warehouse – Officer / Executive
- Sections: RM/PM Receipt, QMS, Inventory Management
- Qualification: B.Pharm, B.Sc
- Experience: 2–6 years in pharma warehousing
- Vacancies: ~3–5 expected
- Responsibilities:
- Manage RM/PM receipt and dispensing per BMR.
- Oversee inventory using SAP/ERP systems.
- Ensure GMP and GDP compliance.
- Skills: Knowledge of SAP, GDP, inventory tools; organizational skills.
- Salary: ₹2–5 Lakhs per annum (Indeed).
6. IT-Computer System Validation (CSV) – Officer / Executive
- Qualification: B.E, B.Tech, MCA, BCA
- Experience: 2–5 years in pharma IT-CSV
- Vacancies: ~2–4 expected
- Responsibilities:
- Execute CSV protocols for PLC-based equipment.
- Review data backups and manage QMS (deviations, CAPA).
- Prepare for USFDA audits.
- Skills: Expertise in CSV, GAMP 5, 21 CFR Part 11; documentation.
- Salary: ₹3–6 Lakhs per annum (Indeed).
Eligibility Criteria
| Category | Details |
|---|---|
| Education | M.Pharm, B.Pharm, B.Sc, M.Sc, Diploma, ITI (Production/QA/QC/Warehouse); B.E, B.Tech, Diploma, ITI (Engineering); B.E, B.Tech, MCA, BCA (IT-CSV) |
| Experience | 0–10 years (Production/Engineering: 0–8; QA: 2–10; QC: 1–5; Warehouse: 2–6; IT-CSV: 2–5); ITI freshers eligible |
| Skills | Production: FBD, HATA; Engineering: PLC; QA: SAP; QC: HPLC; Warehouse: GDP; IT-CSV: GAMP 5 |
| Other Requirements | Shift work; USFDA/MHRA exposure preferred; Surendranagar-based |
Why These Roles?
- Career Growth: 70% of freshers secure permanent roles in 2 years (Emcure data).
- Global Standards: Join a USFDA-compliant OSD plant with zero 483s (2024).
- Skill Development: Master HPLC, SAP, or CSV; 66% rate learning ≥4/5.
- Stable Employer: Emcure supports 8,600+ employees, though management needs updates.
How to Prepare for the Walk-In
- Bring Documents: CV, photo, CTC proof, certificates, experience letters, payslips, Aadhaar/PAN copy.
- Dress Professionally: Wear formal attire.
- Research Emcure: Review www.emcure.com for OSD products (e.g., Metformin) and cGMP standards.
- Prepare Questions: Expect queries on HPLC, IPQA, PLC, SAP, or CSV protocols. Freshers highlight projects.
- Arrive Early: Reach by 8:45 AM; expect 1–2 hour wait (AmbitionBox).
- Online Option: Email CV to atish.patel@emcure.com if unable to attend.
Why Surendranagar, Gujarat?
Surendranagar, a growing pharma hub, hosts Emcure’s OSD plant in Kadu. Gujarat’s $7 billion pharma sector grows at 12% CAGR (Invest India). Connected via NH-47, Surendranagar offers affordable living, ideal for pharma careers, though amenities are limited (AmbitionBox).
Contact Information
- Email: atish.patel@emcure.com
- Phone: +91-2717-610-000
- Venue Address: Emcure Pharmaceuticals Ltd, New Survey No. 485, Village Kadu, Taluka Lakhtar, Surendranagar, Gujarat 382775
- Corporate Office: Emcure Pharmaceuticals Ltd, Plot No. P-2, IT-BT Park, Phase II, MIDC, Hinjewadi, Pune, Maharashtra 411057
- Website: www.emcure.com
- LinkedIn: Emcure Pharmaceuticals
Join Emcure on May 9, 2025, for Cure Beyond in Surendranagar’s OSD plant. Apply with passion for a rewarding career!


