Eugia Pharma Specialities Ltd., a subsidiary of Aurobindo Pharma and a USFDA-approved leader in injectables and oncology, is hosting a walk-in interview for Quality Assurance (QA-IPQA) and Production roles at our Unit-III facility in Pashamylaram. Empower your career with us and contribute to global healthcare innovation!
Contents
Event Details
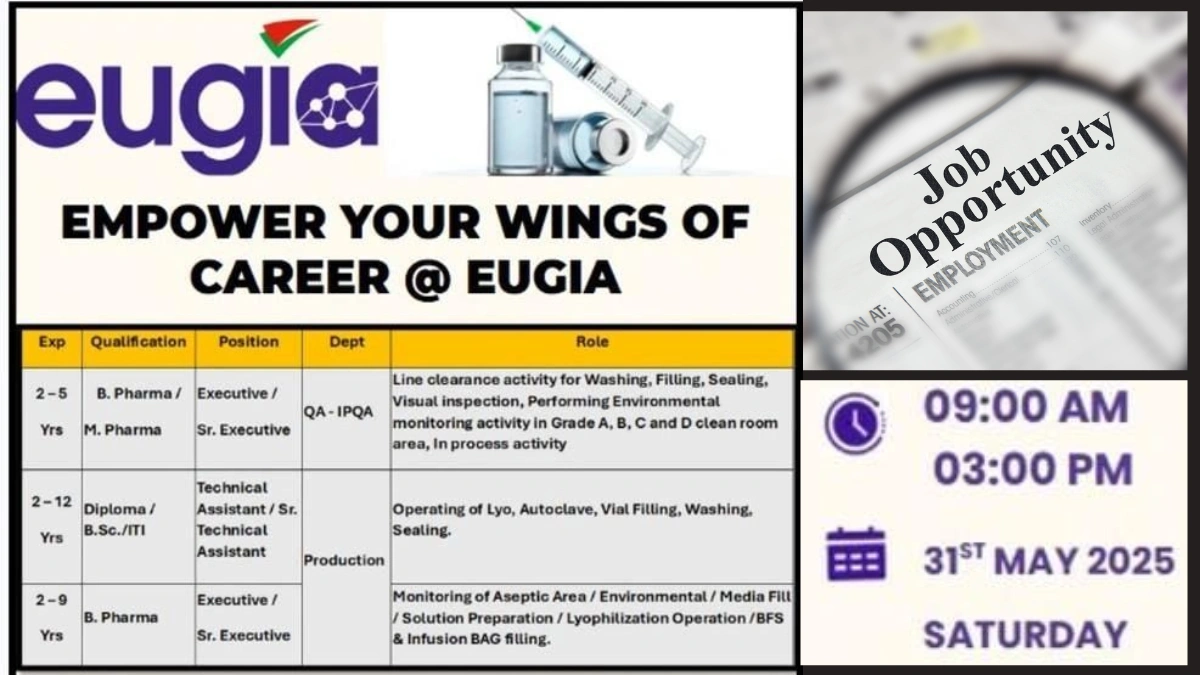
Date: Saturday, May 31, 2025
Time: 9:00 AM to 3:00 PM
Venue: Eugia Pharma Specialities Ltd., Unit-III, Plot No. 4, 34 to 48, EPIP, TSIIC, IDA-Pashamylaram, Patancheru (Mandal), Sangareddy, Telangana 502307
Contact: Email: hr.unit3@eugiapharma.com
Join our dynamic team at a state-of-the-art manufacturing facility.
Open Positions
We’re hiring for Quality Assurance (QA-IPQA) and Production departments. Below are the details:
| Department | Position | Qualification | Experience | Key Responsibilities |
|---|---|---|---|---|
| QA-IPQA | Executive/Sr. Executive | B.Pharm., M.Pharm. | 2-5 Years | Line clearance, environmental monitoring, in-process checks |
| Production | Technical Assistant/Sr. Technical Assistant | Diploma, B.Sc., ITI | 2-12 Years | Operate Lyo, autoclave, vial filling, washing, sealing |
| Production | Executive/Sr. Executive | B.Pharm. | 2-9 Years | Monitor aseptic areas, media fills, solution prep, BFS |
Quality Assurance (QA-IPQA)
- Responsibilities:
- Perform line clearance for washing, filling, sealing, and visual inspection.
- Conduct environmental monitoring in Grade A, B, C, and D cleanroom areas.
- Execute in-process quality checks to ensure compliance with GMP standards.
- Preferences: Experience in USFDA-regulated injectable facilities, knowledge of QMS.
Production
- Technical Assistant/Sr. Technical Assistant:
- Operate equipment including lyophilizers, autoclaves, vial filling, washing, and sealing machines.
- Maintain documentation and ensure aseptic practices.
- Executive/Sr. Executive:
- Monitor aseptic areas, environmental conditions, and media fill activities.
- Oversee solution preparation, lyophilization, BFS, and infusion bag filling operations.
- Preferences: Familiarity with injectable manufacturing, aseptic techniques, and regulatory audits.
Candidate Requirements
- Qualifications: B.Pharm., M.Pharm., Diploma (Mechanical/Electrical), B.Sc., ITI (Fitter/Electrical).
- Experience: 2-5 years (QA-IPQA), 2-12 years (Production Technical Assistant), 2-9 years (Production Executive).
- Preferences: Experience in USFDA/MHRA-regulated plants, proficiency in GMP documentation, and aseptic operations.
- Work Location: Pashamylaram, Telangana.
Documents Required
- Updated resume
- Recent increment letter
- Last 3 months’ payslips
- Last 3 months’ bank statements
- Educational certificates
- Copies of Aadhar and PAN card
Why Join Eugia Pharma Specialities?
Eugia Pharma, part of Aurobindo Pharma, employs over 4,000 staff globally and specializes in injectables, oncology, and ophthalmics. Rated 4.1/5 on AmbitionBox for job security (4.5/5), our Pashamylaram Unit-III facility offers a collaborative environment with a 3.8/5 work-life balance rating. Despite a 2024 FDA warning for CGMP violations, we are committed to quality improvements. Learn more at Eugia Pharma’s website.
How to Apply
Attend the walk-in interview on May 31, 2025, from 9:00 AM to 3:00 PM at Eugia Pharma Specialities Ltd., Unit-III, Pashamylaram. Bring the required documents listed above. Outstation candidates can email resumes to hr.unit3@eugiapharma.com, mentioning “Walk-In – [Role]” in the subject line.