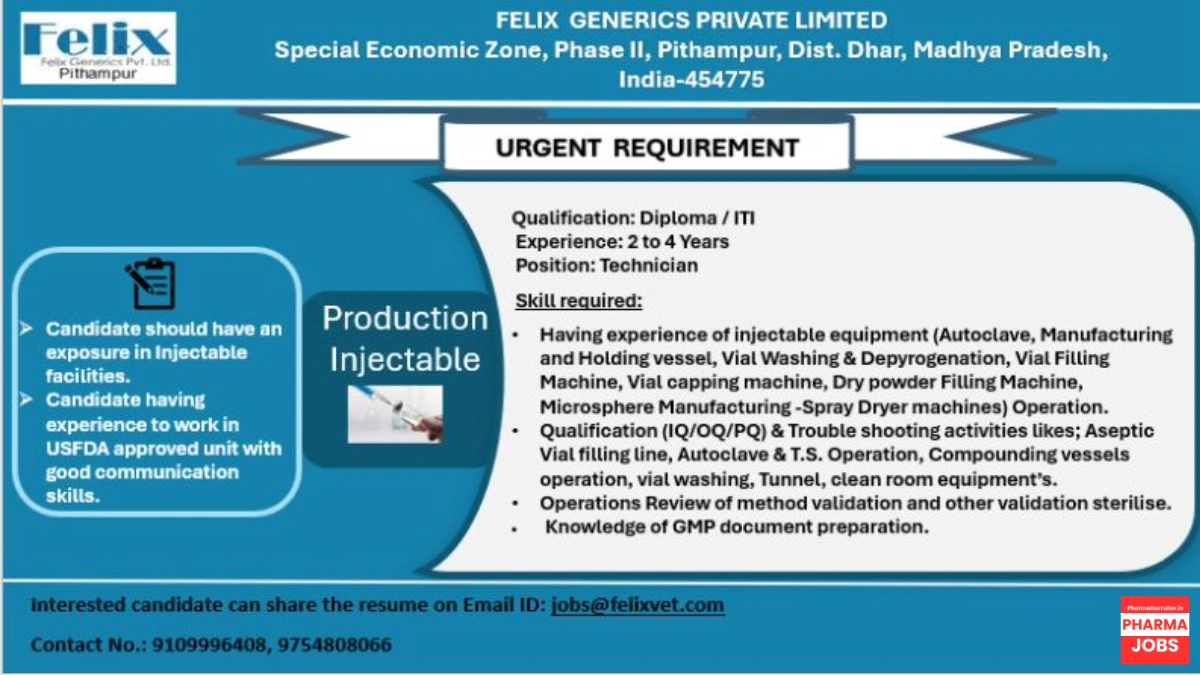
Felix Generics Private Limited, a USFDA-approved pharmaceutical company specializing in veterinary generic medicines, is urgently hiring Technicians for our Production Injectable department at our state-of-the-art facility in Pithampur, Madhya Pradesh. Located in the Special Economic Zone, Phase II, our HPRA-approved (April 2021) plant supports global markets with a focus on sterile injectables. Join our team to contribute to high-quality pharmaceutical manufacturing in a cGMP-compliant environment!
Contents
Walk in Drive Details
Date: 23rd May, 2025
Venue: Special Economic Zone, Phase II, Pithampur, Dist. Dhar, Madhya Pradesh, India – 454775
Open Position: Technician (Production Injectable)
Department
- Production Injectable
Qualification
- Diploma or ITI (Mechanical, Electrical, Pharmaceutical, or related fields)
Experience
- 2–4 years in injectable facilities
Position
- Technician
Location
- Special Economic Zone, Phase II, Pithampur, Dist. Dhar, Madhya Pradesh, India – 454775
Skills Required
- Equipment Operation: Hands-on experience with injectable equipment, including:
- Autoclave, Manufacturing and Holding Vessels, Vial Washing & Depyrogenation, Vial Filling Machine, Vial Capping Machine, Dry Powder Filling Machine, and Microsphere Manufacturing (Spray Dryer).
- Qualification Activities: Perform Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) for:
- Aseptic Vial Filling Line, Autoclave & Terminal Sterilization (T.S.), Compounding Vessels, Vial Washing, Tunnel, and Clean Room Equipment.
- Troubleshooting: Address operational issues in aseptic processing and sterile manufacturing.
- Validation Review: Support review of method validation and sterilization validation protocols.
- GMP Documentation: Prepare and maintain Good Manufacturing Practice (GMP) documents, including SOPs, batch records, and qualification reports.
- Regulatory Exposure: Experience working in a USFDA-approved facility with adherence to cGMP standards.
- Communication: Strong verbal and written communication skills for cross-functional coordination.
Candidate Requirements
- Eligibility:
- Candidates with 2–4 years of experience in injectable manufacturing in a USFDA-approved unit.
- Familiarity with aseptic techniques, clean room operations, and sterile processing.
- Documents to Submit:
- Updated resume highlighting relevant experience.
- Photocopies of educational certificates (Diploma/ITI).
- Aadhar card, PAN card, and one passport-size photograph.
- Last 3 months’ pay slips or CTC proof (if applicable).
How to Apply
- Send Your Resume: Email your updated CV to jobs@felixvet.com with the subject line “Technician – Production Injectable”.
- Contact: Call +91-9109996408 or +91-9754808066 for queries or to schedule an interview.
- Company Website: Felix Generics


Why Join Felix Generics Pvt. Ltd.?
- Global Reach: Part of an Ireland-based parent entity, our Pithampur facility supports veterinary generics for international markets, with a corporate office in Gurgaon and R&D center in Greater Noida.
- Advanced Facility: Work in a USFDA and HPRA-approved plant equipped for sterile injectables, including vial filling and microsphere manufacturing.
- Career Development: Opportunities to gain expertise in aseptic processing, equipment qualification, and GMP compliance.
- Supportive Environment: Collaborative team culture with a focus on innovation and quality in pharmaceutical manufacturing.
Important Notes
- No Recruitment Fees: Felix Generics does not charge fees for recruitment. Report suspicious activities to jobs@felixvet.com.
- Application Tip: Highlight experience with injectable equipment and GMP documentation in your resume to stand out.
Apply now to join Felix Generics Pvt. Ltd. and advance your career in pharmaceutical injectable jobs in Pithampur! Email your resume to jobs@felixvet.com or contact +91-9109996408 / +91-9754808066.