Unlock QC jobs at Finecure! Attend walk-in interviews for pharma jobs in HPLC and instrumentation at this USFDA, EU-GMP certified leader. Advance pharmaceutical careers in India on October 12.
Contents
About the Company
Finecure Pharmaceuticals Limited, established in 2005 and headquartered in Ahmedabad, Gujarat, is a premier manufacturer of pharmaceutical formulations across multiple therapeutic segments.
Specializing in tablets, capsules, and dry powders for syrups, the company operates state-of-the-art facilities in Sanand and Changodar, holding USFDA and EU-GMP certifications alongside WHO-GMP and ISO standards for quality, environment, and occupational health.
With a global footprint in the USA, Europe, Southeast Asia, and Latin America, Finecure drives innovation through rigorous R&D and regulatory compliance, ensuring high product quality and sustainable growth in the competitive pharma landscape.
Job Details
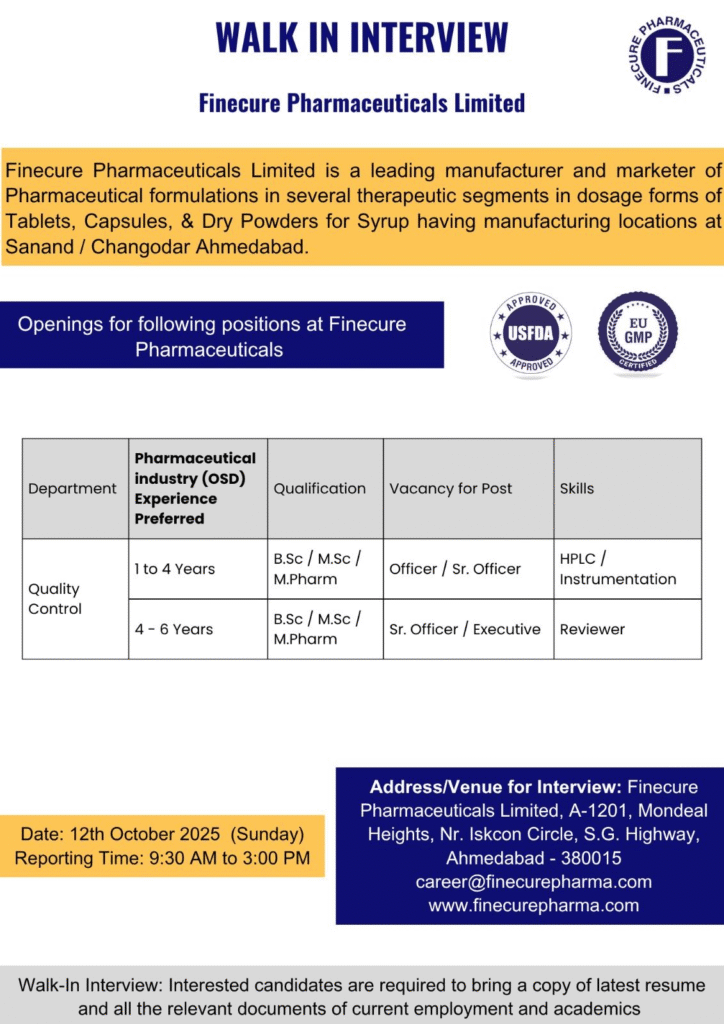
- Company Name: Finecure Pharmaceuticals Limited
- Experience: 1 to 6 Years (varies by role)
- Qualification: B.Sc / M.Sc / M.Pharm
- Location: Sanand / Changodar, Ahmedabad, Gujarat
- Work Type: On-site
Job Description
Finecure Pharmaceuticals is expanding its quality control team with immediate pharma jobs in OSD formulations. This walk-in drive seeks experienced professionals for analytical and review roles. Leverage your expertise in pharmaceutical careers in India at a USFDA-approved facility.
Officer / Sr. Officer
- Department: Quality Control
- Role: HPLC and Instrumentation Analysis
- Experience: 1 to 4 Years
- Education/Qualification: B.Sc / M.Sc / M.Pharm
Sr. Officer / Executive (Reviewer)
- Department: Quality Control
- Role: Document and Data Review
- Experience: 4 to 6 Years
- Education/Qualification: B.Sc / M.Sc / M.Pharm
Skills/Qualifications
- Proficiency in HPLC operations and instrumental techniques
- Strong knowledge of wet chemistry and analytical methods
- Experience in document review and quality data verification
- OSD pharmaceutical industry background preferred
- Familiarity with USFDA and EU-GMP compliance standards
- M.Pharm or M.Sc. in relevant sciences for advanced roles
Key Responsibilities
- Perform HPLC assays for raw materials
- Handle instrumentation for quality testing
- Review analytical data for compliance
- Prepare QC reports and SOPs daily
- Support audit readiness activities
- Ensure GLP in lab operations
Benefits/Perks
- Career progression in global regulatory environments
- Ongoing training in advanced pharma technologies
- Competitive salaries with performance incentives
- Inclusive culture at USFDA-certified facilities
- Exposure to international markets and R&D
How to Apply
Walk in with your latest resume and relevant documents for on-site screening. Alternatively, email your CV to career@finecurepharma.com with subject “QC Application – [Position]“.
Verified Post
The post is released by the Finecure Pharmaceuticals LinkedIn page. Click here to visit the post
For resume optimization in pharma jobs, visit Pharma Recruiter. Apply today—elevate your pharmaceutical careers in India!
Walk-in Interview Details
- Date: 12th October 2025 (Sunday)
- Time: 9:30 AM to 3:00 PM
- Venue: Finecure Pharmaceuticals Limited, A-1201, Mondeal Heights, Nr. Iskcon Circle, S.G. Highway, Ahmedabad – 380015
- Contact/Email: Email: career@finecurepharma.com; Website: www.finecurepharma.com
- What to Bring: Latest resume, current employment and academic documents
Why You Should Join
Finecure Pharmaceuticals champions a culture of excellence and innovation, recognized for its USFDA and EU-GMP certifications that guarantee stability in QC roles. Since 2005, enjoy long-term growth through R&D advancements and global exports, in a compliance-focused ecosystem that rewards analytical expertise and fosters professional development for lasting impact in healthcare.
FAQs
What experience is ideal for QC jobs at Finecure?
1-6 years in OSD pharma, with HPLC/instrumentation skills; prior regulatory exposure boosts chances for reviewer roles.
How does the walk-in process work?
Arrive between 9:30 AM-3:00 PM on October 12 with documents for direct interviews at the Ahmedabad venue.
Are OSD skills mandatory for these pharma jobs?
Preferred for all positions to align with tablet/capsule production in pharmaceutical careers in India.
What growth opportunities await?
From officer to executive, with training in USFDA compliance and paths to senior QC leadership.


