Are you a pharmaceutical professional with OSD experience? Finecure Pharmaceuticals Limited, a leader in formulations, hosts walk-in interviews on September 21, 2025. Join our USFDA and EU GMP audited facilities in Sanand/Changodar, Ahmedabad, to innovate in healthcare.
Contents
- 1 About Finecure Pharmaceuticals Limited
- 2 Why Join Finecure’s Dynamic Team?
- 3 Walk-in Interview Details
- 4 Open Positions in Quality Control
- 5 Open Positions in Quality Assurance
- 6 Open Positions in Formulation R&D
- 7 Open Positions in Supply Chain Management
- 8 Required Documents and Eligibility
- 9 Employee Benefits at Finecure
- 10 How to Prepare for the Interview
- 11 Why Ahmedabad for Pharma Careers?
- 12 Frequently Asked Questions
- 13 Company Certifications and Growth
- 14 Final Call to Action
About Finecure Pharmaceuticals Limited
Established in 2005, Finecure is the flagship of Chandrans Group. We manufacture tablets, capsules, dry powders for syrups, oral liquids, injections, and sachets. Our focus is niche therapeutics like cardiovascular and neurology.
Our Ahmedabad-based plants ensure global quality standards. With a presence in India and overseas, we drive healthier lives through innovative products. Visit www.finecurepharma.com for insights.
Why Join Finecure’s Dynamic Team?
At Finecure, we blend entrepreneurship with strong ethics. Our WHO-GMP certified units offer growth in a collaborative environment. Contribute to therapeutic advancements while advancing your career.
Enjoy competitive benefits, training, and exposure to international standards. Be part of a company recognized for business excellence awards.
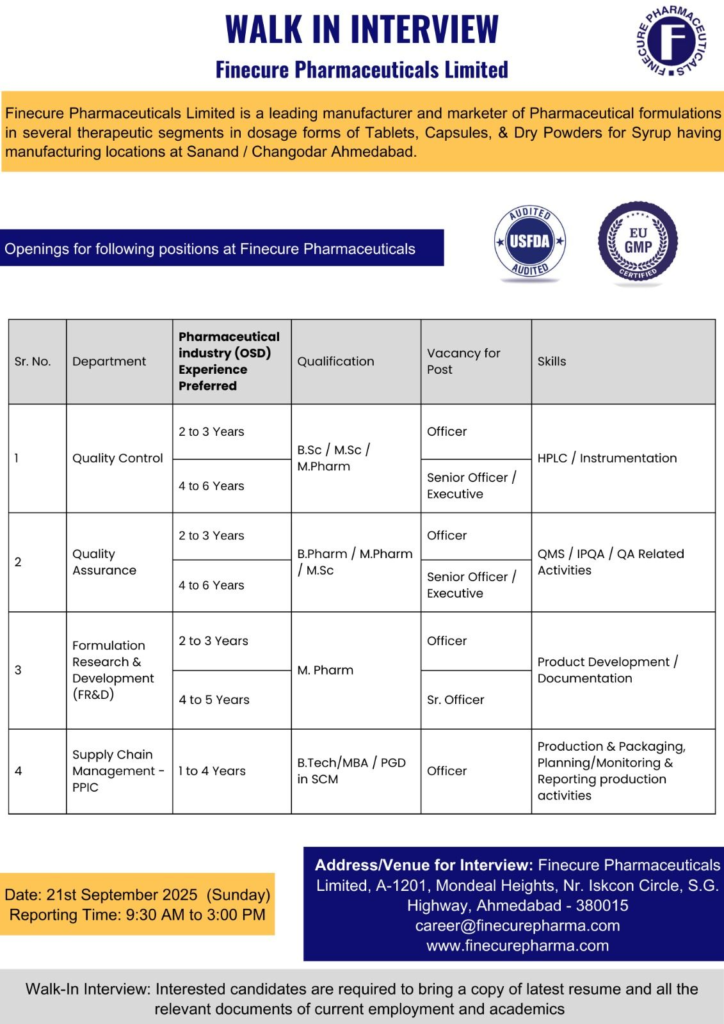
Walk-in Interview Details
Mark your calendar for this exclusive hiring event in Ahmedabad. It’s tailored for experienced OSD professionals.
- Date: Sunday, 21st September 2025
- Time: 9:30 AM to 3:00 PM
- Venue: Finecure Pharmaceuticals Limited, A-1201, Mondeal Heights, Nr. Iskcon Circle, S.G. Highway, Ahmedabad – 380015
Bring your resume and documents. Email CVs to career@finecurepharma.com for pre-screening.
Open Positions in Quality Control
We’re expanding our QC team to maintain rigorous standards. Focus on analytical excellence in OSD formulations.
QC Officer
Conduct routine testing and instrumentation analysis. Ensure compliance in raw materials and finished products.
Key Responsibilities
- Perform HPLC and instrumental analyses.
- Document results and maintain lab records.
- Support stability studies and method validation.
Qualifications and Experience
- B.Sc/M.Sc/M.Pharm.
- 2-3 years in pharmaceutical QC.
- Skills in HPLC and instrumentation.
Senior Officer/Executive – QC
Lead QC operations and team coordination. Oversee advanced testing protocols.
Key Responsibilities
- Supervise daily lab activities.
- Handle OOS investigations and CAPA.
- Prepare for regulatory audits.
Qualifications and Experience
- B.Sc/M.Sc/M.Pharm.
- 4-6 years in QC with OSD focus.
- Expertise in analytical techniques.
Open Positions in Quality Assurance
Strengthen our QA framework with experienced hires. Emphasize QMS and IPQA activities.
QA Officer
Execute in-process checks and documentation. Support manufacturing compliance.
Key Responsibilities
- Perform IPQA during production.
- Review batch records and SOPs.
- Ensure line clearance and validation.
Qualifications and Experience
- B.Pharm/M.Pharm/M.Sc.
- 2-3 years in QA.
- Knowledge of QMS activities.
Senior Officer/Executive – QA
Drive quality systems and audit readiness. Mentor junior staff.
Key Responsibilities
- Implement QMS and CAPA processes.
- Conduct internal audits.
- Liaise with regulatory bodies.
Qualifications and Experience
- B.Pharm/M.Pharm/M.Sc.
- 4-6 years in pharmaceutical QA.
- Proficiency in IPQA and GMP.
Open Positions in Formulation R&D
Innovate in our FR&D department. Develop new OSD formulations for therapeutic segments.
FR&D Officer
Assist in product development and lab-scale trials. Focus on documentation accuracy.
Key Responsibilities
- Conduct formulation experiments.
- Prepare stability and tech transfer docs.
- Scale-up prototypes.
Qualifications and Experience
- M.Pharm.
- 2-3 years in FR&D.
- Skills in product development.
Senior Officer – FR&D
Lead R&D projects and innovation pipelines. Collaborate with production teams.
Key Responsibilities
- Design and optimize formulations.
- Manage documentation and IP filings.
- Ensure regulatory compliance.
Qualifications and Experience
- M.Pharm.
- 4-5 years in OSD R&D.
- Expertise in documentation.
Open Positions in Supply Chain Management
Optimize our SCM operations for efficient production planning. Monitor OSD workflows.
PPIC Officer
Plan and track production schedules. Report on packaging and inventory.
Key Responsibilities
- Develop production plans.
- Monitor activities and resolve delays.
- Generate performance reports.
Qualifications and Experience
- B.Tech/MBA/PGD in SCM.
- 1-4 years in pharma SCM.
- Focus on planning and reporting.
Required Documents and Eligibility
All roles prefer OSD experience in pharmaceuticals. Bring photocopies of:
- Latest resume.
- Educational certificates.
- Current employment docs (payslips, relieving letter).
Gujarat-based candidates prioritized. Pharmaceutical industry background mandatory.
Employee Benefits at Finecure
We offer attractive packages including health insurance and transport. Enjoy professional growth in a certified environment.
Our culture promotes innovation and teamwork. Access training in GMP and regulatory affairs.
Key Perks
- Competitive salaries and incentives.
- Skill development programs.
- Supportive work-life balance.
How to Prepare for the Interview
Tailor your profile to OSD expertise. Review USFDA guidelines. Practice discussing past QA or R&D projects.
Verified Post
The post is released by the Finecure Pharmaceuticals Limited LinkedIn page. Click here to visit the post
Arrive early and dress professionally. Highlight GMP knowledge.
Interview Tips
- Showcase analytical skills for QC roles.
- Discuss QMS implementations for QA.
- Prepare SCM planning examples.
Why Ahmedabad for Pharma Careers?
Ahmedabad is Gujarat’s pharma hub with robust infrastructure. Finecure’s facilities drive exports in pharmaceutical formulations.
Join a thriving ecosystem for career advancement.
Frequently Asked Questions
-
What is the reporting time?
9:30 AM to 3:00 PM on September 21, 2025.
-
Are freshers eligible?
No, minimum 1-6 years’ experience required.
-
What certifications matter?
USFDA/EU GMP knowledge is a plus.
Company Certifications and Growth
Our plants are USFDA audited and EU GMP certified. We excel in cardiovascular and anti-infectives segments.
With 20 years of excellence, Finecure leads in nutraceuticals too.
Final Call to Action
Don’t miss this opportunity to join Finecure Pharmaceuticals. Attend the walk-in tomorrow and email career@finecurepharma.com. Innovate in OSD formulations with us.
| Sr. No. | Department | Position | Experience | Qualifications | Skills |
|---|---|---|---|---|---|
| 1 | Quality Control | Officer | 2-3 Years | B.Sc/M.Sc/M.Pharm | HPLC/Instrumentation |
| 2 | Quality Control | Senior Officer/Executive | 4-6 Years | B.Sc/M.Sc/M.Pharm | HPLC/Instrumentation |
| 3 | Quality Assurance | Officer | 2-3 Years | B.Pharm/M.Pharm/M.Sc | QMS/IPQA/QA Activities |
| 4 | Quality Assurance | Senior Officer/Executive | 4-6 Years | B.Pharm/M.Pharm/M.Sc | QMS/IPQA/QA Activities |
| 5 | FR&D | Officer | 2-3 Years | M.Pharm | Product Development/Documentation |
| 6 | FR&D | Sr. Officer | 4-5 Years | M.Pharm | Product Development/Documentation |
| 7 | Supply Chain – PPIC | Officer | 1-4 Years | B.Tech/MBA/PGD in SCM | Production Planning/Monitoring |
- Global exposure in audited facilities.
- Career progression in therapeutics.
- Inclusive and innovative culture.
- Impactful roles in healthcare.
We look forward to welcoming you!


