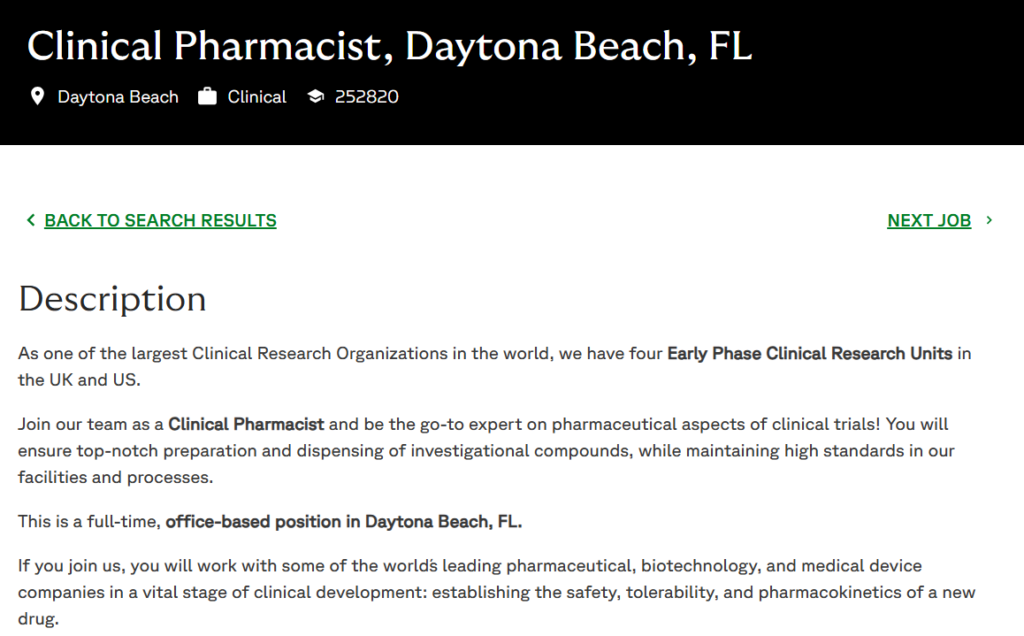
Are you a registered pharmacist eager to advance clinical trials for groundbreaking therapies? Fortrea, a global leader in contract research organizations (CROs), is hiring a full-time Clinical Pharmacist in Daytona Beach, FL (Job ID: 252820).
Join our Early Phase Clinical Research Unit to ensure precise preparation and dispensing of investigational compounds, supporting pharmaceutical, biotech, and medical device innovators.
With operations in over 90 countries and more than 19,000 staff, Fortrea drives drug and device development across 20+ therapeutic areas. Explore our passion for scientific rigor at www.fortrea.com.
Contents
- 1 About Fortrea: Transforming Clinical Development Worldwide
- 2 Role Overview: Clinical Pharmacist in Early Phase Trials
- 3 Key Responsibilities: Drive Trial Success Through Expertise
- 4 Qualifications: What We’re Seeking
- 5 Why Join Fortrea’s Daytona Beach Team?
- 6 Benefits: Comprehensive Support for Full-Time Employees
- 7 How to Apply: Secure Your Clinical Pharmacist Role
- 8 Ready to Elevate Your Pharma Career at Fortrea?
About Fortrea: Transforming Clinical Development Worldwide
Fortrea is a premier CRO focused on overcoming barriers in clinical trials through innovative solutions in patient access, technology, and development. Our four Early Phase units in the UK and US, including Daytona Beach, specialize in establishing safety, tolerability, and pharmacokinetics for new drugs.
Our Fortrea FOUR culture—Forward Together, Own It, Uphold Integrity, Respect People—fosters collaboration and growth. As a Great Place to Work-certified employer, we prioritize diversity and employee impact. Learn more on our LinkedIn page.
The global CRO market is expanding rapidly, creating abundant pharma jobs in clinical pharmacology. Stay informed via PharmaVoice.
Role Overview: Clinical Pharmacist in Early Phase Trials
This office-based, full-time position in the Clinical category demands expertise in a fast-paced, protocol-driven environment. You’ll serve as the pharmaceutical authority, coordinating with sponsors and regulatory bodies to maintain compliance and excellence.
Key focus: High standards in sterile compounding and dispensing for Phase I trials. This role suits those with hospital, long-term care, or research pharmacy backgrounds, targeting keywords like “clinical pharmacist jobs Florida” and “pharma CRO careers Daytona Beach.”
Key Responsibilities: Drive Trial Success Through Expertise
Leverage your skills to support clinical teams and ensure seamless operations in our Daytona Beach facility.
Core Duties
- Coordinate with sponsors to demonstrate pharmacy and dispensing capabilities
- Develop and review study protocols, pharmacy manuals, and preparation logs
- Engage with regulatory bodies for pharmacy department compliance
- Advise clinical teams and departments on technical pharmaceutical matters
- Maintain facilities and processes per current regulations and guidelines
- Improve pharmacy equipment and procedures for efficiency
- Conduct feasibility reviews for new proposals
- Implement standard processes to uphold good dispensing practices
- Handle all other duties as assigned
These responsibilities align with GxP standards like GMP and GCP. For insights into similar roles, visit Indeed’s Fortrea listings.
Qualifications: What We’re Seeking
Fortrea values equivalent experience, making this accessible for skilled professionals.
Essential Requirements
- Registered Pharmacist or PharmD per Florida state requirements
- 2+ years of pharmacy experience, including sterile compounding
- Preferred: Direct experience in research, hospital, or long-term care settings
- Comfort in a fast-paced, no-deviation environment with shifting priorities
- Strong teamwork, people skills, and technology proficiency (electronic data collection)
Fortrea may substitute relevant experience for formal education. Ideal candidates excel in pharmacology, pharmacokinetics, and clinical decision-making.
Why Join Fortrea’s Daytona Beach Team?
Work in a collaborative, technology-driven setting where your expertise directly impacts global therapies. Our Daytona Beach unit (1900 Mason Avenue, Suite 140) offers close participant interaction in a vibrant Florida location.
Benefit from exposure to leading companies and a supportive culture. With 6+ local openings, including research nurses and technicians, career growth is plentiful. Explore trends in early-phase trials via Express Pharma.
Benefits: Comprehensive Support for Full-Time Employees
Eligible full-time staff (20+ hours/week) enjoy robust perks to enhance well-being and security.
Key Benefits
- Medical, Dental, Vision, Life, STD/LTD insurance (multiple carriers)
- 401(K) retirement plan
- Paid Time Off (PTO)
- Employee recognition awards
- Multiple Employee Resource Groups (ERGs)
We prioritize EEO and accommodations—learn more here. Salaries range from $113,800–$135,800 annually, based on experience.
How to Apply: Secure Your Clinical Pharmacist Role
Ready to contribute to innovative trials? Applications are open—apply swiftly for this Daytona Beach opportunity.
Application Steps
- Visit Fortrea’s careers portal and search for Job ID: 252820
- Submit your resume, PharmD certification, and cover letter
- Highlight sterile compounding and research experience
- For queries, email recruiting@fortrea.com
Verified Clinical Research Job post
The post is released by the Fortrea Career page. Click here to visit the post
Tailor your profile to emphasize GxP knowledge and adaptability. Share on LinkedIn.
Tips for Success
- Quantify achievements, e.g., “Managed 50+ compounding protocols”
- Prepare for interviews on regulatory compliance and protocol reviews
- Research Fortrea’s Phase I focus for insightful questions
Ready to Elevate Your Pharma Career at Fortrea?
Join our Daytona Beach team and shape the future of clinical development. This Clinical Pharmacist role offers stability, impact, and growth in a leading CRO.
Apply now via our portal—your expertise is needed to advance life-changing therapies!