Are you ready to advance your career in the pharmaceutical industry? Gracure Pharmaceuticals Limited, a trusted name in pharmaceutical manufacturing since 1992, is hosting a hiring drive for skilled professionals to join our EU-GMP-approved facility in Bhiwadi, Rajasthan.
With a focus on innovation and affordable medicines, Gracure offers a dynamic workplace for those passionate about quality and excellence in pharmaceuticals. Apply now and become part of our mission to deliver world-class healthcare solutions!
Contents
About Gracure Pharmaceuticals Limited
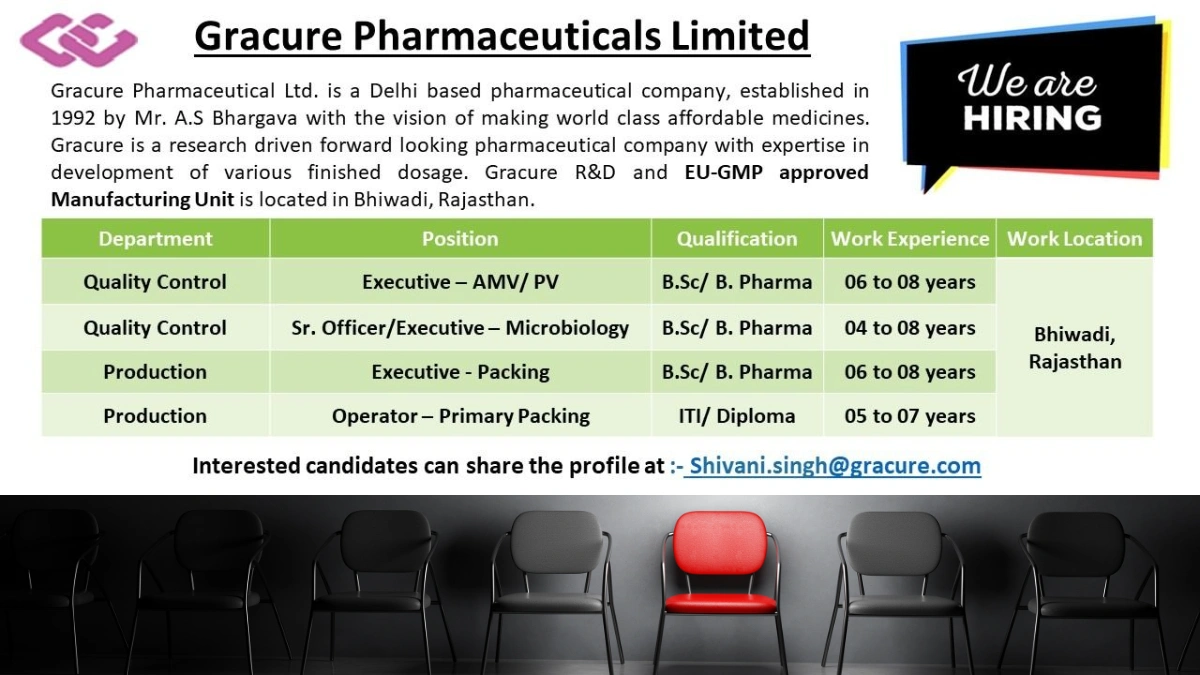
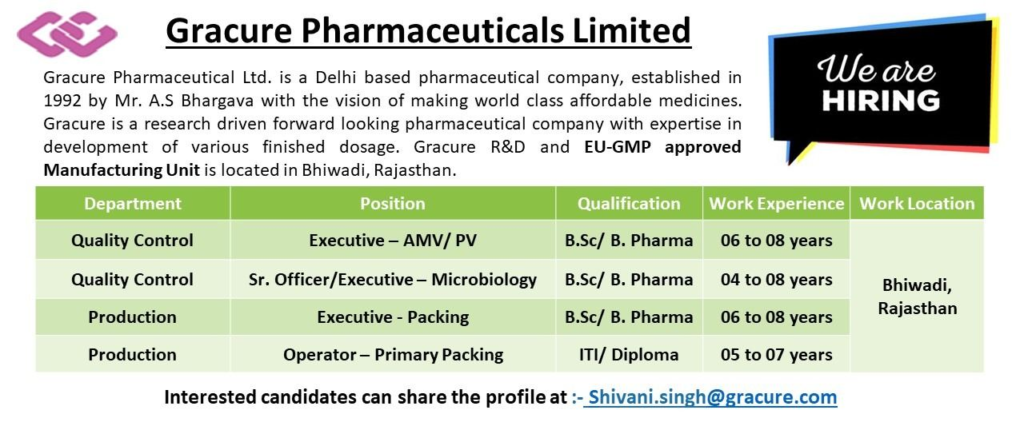
Founded in 1992 by Mr. A.S Bhargava, Gracure Pharmaceuticals Limited is a Delhi-based pharmaceutical company dedicated to producing world-class, affordable medicines. With a strong emphasis on research and development, Gracure specializes in various finished dosage forms and operates an EU-GMP and TGA-approved manufacturing unit in Bhiwadi, Rajasthan.
Our commitment to Good Manufacturing Practices (GMP) and innovation positions us as a leader in the global pharmaceutical industry.
We are hiring experienced professionals for our Quality Control and Production departments. If you have expertise in pharmaceutical manufacturing, this is your opportunity to grow with Gracure!
Job Location
- Bhiwadi, Rajasthan
Available Positions
Explore our open roles in Quality Control and Production. Below is a user-friendly breakdown of the positions, qualifications, experience requirements, and job responsibilities. All roles require relevant experience in pharmaceutical manufacturing.
1. Quality Control – Executive (AMV/PV)
- Experience: 6-8 years
- Qualification: B.Sc., B.Pharm
- Key Skills:
- Expertise in Analytical Method Validation (AMV) and Process Validation (PV)
- Proficiency with analytical instruments such as HPLC and GC
- Knowledge of ICH guidelines and pharmacopoeial standards
- Familiarity with SOPs, STPs, and documentation for regulatory compliance
- What You’ll Do:
- Conduct analytical method validation and process validation activities
- Prepare and review validation protocols and reports
- Ensure compliance with GMP and regulatory requirements
- Support quality control testing and documentation
2. Quality Control – Senior Officer/Executive (Microbiology)
- Experience: 4-8 years
- Qualification: B.Sc., B.Pharm
- Key Skills:
- Expertise in microbiological testing, including MLT, bioburden, and sterility testing
- Knowledge of environmental monitoring and water analysis
- Familiarity with GMP, GLP, and data integrity
- Experience with trend data preparation and QC investigations
- What You’ll Do:
- Perform microbiological testing of raw materials, finished products, and stability samples
- Conduct environmental monitoring and prepare trend data
- Support QC investigations (OOS, OOT, lab incidents)
- Ensure compliance with quality and regulatory standards
3. Production – Executive (Packing)
- Experience: 6-8 years
- Qualification: B.Sc., B.Pharm
- Key Skills:
- Expertise in primary and secondary packing operations for tablets, capsules, or other dosage forms
- Knowledge of BMR/BPR review, in-process checks, and QMS
- Experience with labelling machines, track-and-trace systems, and 2D inspection machines
- Strong understanding of aseptic practices and GMP compliance
- What You’ll Do:
- Oversee packing operations, including primary and secondary packing
- Conduct in-process checks and review BMR/BPR
- Ensure compliance with quality standards and regulatory requirements
- Supervise packing area activities and maintain documentation
4. Production – Operator (Primary Packing)
- Experience: 5-7 years
- Qualification: ITI, Diploma
- Key Skills:
- Hands-on experience in primary packing operations for pharmaceutical products
- Familiarity with packing equipment and track-and-trace systems
- Knowledge of GMP and aseptic practices
- Ability to perform manual visual inspections and ensure quality standards
- What You’ll Do:
- Operate primary packing equipment for tablets, capsules, or other dosage forms
- Perform manual visual inspections and quality checks
- Ensure compliance with BMR/BPR and GMP standards
- Support packing operations and maintain accurate records
Why Join Gracure Pharmaceuticals Limited?
Global Impact:
- Contribute to the production of affordable, high-quality medicines for global markets
- Work in an EU-GMP and TGA-approved facility
Career Growth:
- Gain exposure to advanced pharmaceutical processes and technologies
- Opportunities for professional development in a research-driven environment
Innovation-Driven:
- Be part of a team focused on developing innovative dosage forms
- Support regulatory submissions for markets like EU, UK, Canada, and Australia.
Supportive Culture:
- Join a collaborative workplace with a focus on quality and excellence
- Work in a facility rated 3.6/5 for salary and benefits by employees
How to Apply
Submit Your CV:
- Email your updated resume to: Shivani.singh@gracure.com
- Include details of your pharmaceutical experience, current salary, and notice period
Walk-In Interview:
- While no specific walk-in date is provided, candidates are encouraged to contact Shivani Singh for potential interview opportunities
- Contact: Shivani Singh at Shivani.singh@gracure.com
Note: Only candidates with relevant experience in pharmaceutical manufacturing should apply
For more information about Gracure Pharmaceuticals Limited, visit our website.
Frequently Asked Questions (FAQs)
What qualifications are required for these roles?
Quality Control (AMV/PV, Microbiology): B.Sc., B.Pharm
Production (Packing): B.Sc., B.Pharm
Production (Primary Packing Operator): ITI, Diploma
Is pharmaceutical experience mandatory?
Yes, candidates must have relevant experience in pharmaceutical manufacturing, preferably in API or formulation processes.
What is the work culture like at Gracure Pharmaceuticals?
Gracure is rated 3.7/5 for work culture and 4.1/5 for skill development by employees, indicating a supportive environment with opportunities to learn, though job security is rated lower at 3.6/5.
Can I apply if I can’t attend a walk-in interview?
Yes, email your CV to Shivani.singh@gracure.com with details of your experience and qualifications.
Join Gracure Pharmaceuticals Limited in Bhiwadi, Rajasthan, and take the next step in your pharmaceutical career. Apply today to become part of a research-driven, globally recognized organization dedicated to affordable healthcare solutions!