Hy-Gro Chemicals Pharmtek Pvt. Ltd., a leading USFDA, KFDA, EDQM, and ANVISA-certified manufacturer of bulk drugs and APIs, is hiring for multiple roles at our Bollaram facility in Telangana. Established in the 1970s and operational since 1995, we are a trusted name in Active Pharmaceutical Ingredients (APIs), Advanced Intermediates, and Specialty Fine Chemicals. Join our team to contribute to global pharmaceutical innovation.
Contents
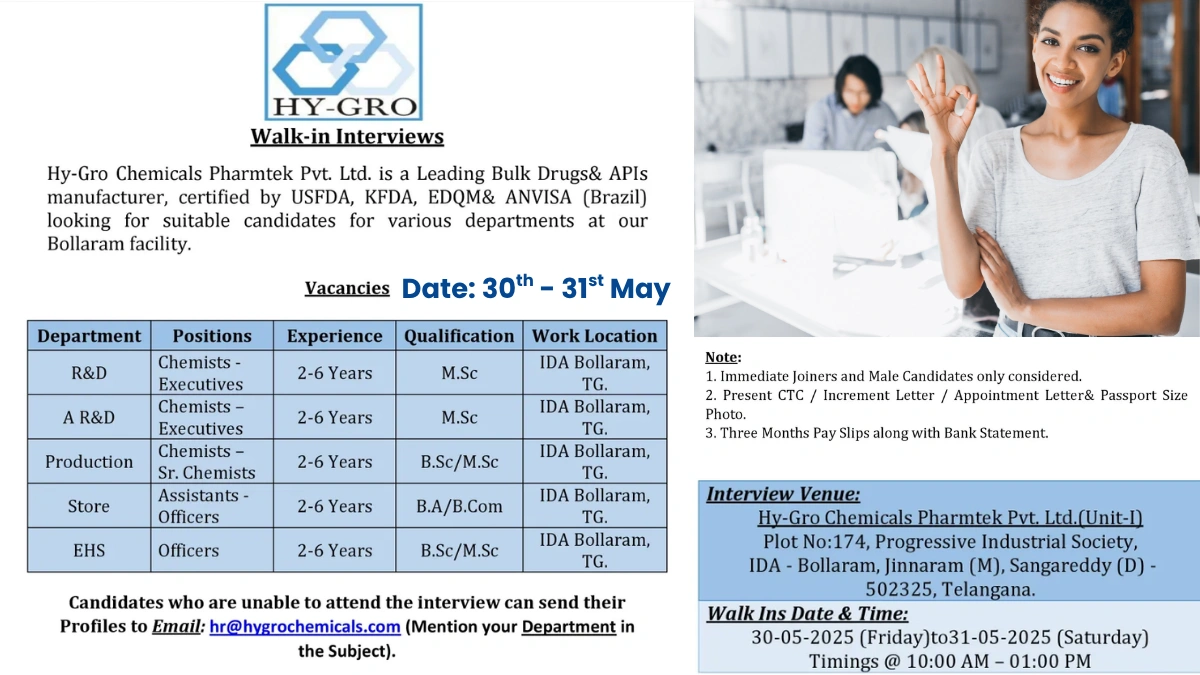
Walk-In Interview Details
Seize this opportunity to advance your career with Hy-Gro Chemicals. Walk-in interviews are scheduled for May 30-31, 2025 (Friday & Saturday) from 10:00 AM to 1:00 PM at Hy-Gro Chemicals Pharmtek Pvt. Ltd. (Unit-I), Plot No: 174, Progressive Industrial Society, IDA – Bollaram, Jinnaram (M), Sangareddy (D) – 502325, Telangana, India. Only male candidates with the ability to join immediately will be considered. Bring your updated CV and required documents to apply.
Open Positions
R&D – Chemists/Executives
- Experience: 2-6 Years
- Qualification: M.Sc.
- Work Location: IDA Bollaram, Telangana
- Responsibilities: Conduct research, develop processes for API synthesis, and maintain lab notebooks.
Analytical R&D (AR&D) – Chemists/Executives
- Experience: 2-6 Years
- Qualification: M.Sc.
- Work Location: IDA Bollaram, Telangana
- Responsibilities: Perform analytical method development, validation, and stability studies.
Production – Chemists/Sr. Chemists
- Experience: 2-6 Years
- Qualification: B.Sc./M.Sc.
- Work Location: IDA Bollaram, Telangana
- Responsibilities: Oversee API manufacturing, ensure process compliance, and optimize production.
Store – Assistants/Officers
- Experience: 2-6 Years
- Qualification: B.A./B.Com.
- Work Location: IDA Bollaram, Telangana
- Responsibilities: Manage inventory, handle material storage, and maintain records.
EHS – Officers
- Experience: 2-6 Years
- Qualification: B.Sc./M.Sc.
- Work Location: IDA Bollaram, Telangana
- Responsibilities: Ensure environmental, health, and safety compliance, and conduct risk assessments.
Why Join Hy-Gro Chemicals Pharmtek?
Hy-Gro Chemicals, with over 50 years of expertise, offers a dynamic work environment at our USFDA-approved Bollaram facility. Join us for:
- Global Impact: Work with a leading API manufacturer serving international markets.
- Career Growth: Opportunities for skill development in a high-compliance environment.
- Innovative Culture: Contribute to cutting-edge pharmaceutical research and production.
- Work-Life Balance: Rated 3.9/5 for work-life balance by employees on AmbitionBox.
Application Process
Attend the walk-in interview with the following documents:
- Updated CV
- Passport-size photo
- Present CTC, increment letter, or appointment letter
- Three months’ payslips with bank statement
- Educational certificates and ID proof
If unable to attend, email your CV to hr@hygrochemicals.com with the subject line mentioning the department (e.g., “R&D Application”). For inquiries, contact HR at hr@hygrochemicals.com. Learn more at Hy-Gro Chemicals.
How to Prepare for the Interview
To stand out, follow these steps:
- Tailor Your CV: Highlight relevant experience in API manufacturing, analytical methods, or EHS compliance.
- Prepare Technical Skills: Be ready to discuss HPLC, GC, or process optimization for R&D/Production roles; inventory management for Store roles; or safety protocols for EHS roles.
- Bring Documents: Ensure all required documents are organized.
- Arrive Early: Demonstrate punctuality and professionalism.
About Hy-Gro Chemicals Pharmtek
Founded in the 1970s, Hy-Gro Chemicals Pharmtek Pvt. Ltd. began as a trading company and started manufacturing APIs in 1995 at its Bollaram facility (Unit-I). Certified by USFDA, KFDA, EDQM, and ANVISA, we produce high-quality APIs, Advanced Intermediates, and Specialty Fine Chemicals. With a revenue of INR 100-500 Crore and a 3.8/5 employee rating, we are a trusted employer in Telangana. Join us to drive pharmaceutical excellence.