Indoco Remedies is hiring experienced professionals for QC and stability roles at its Waluj facility. Walk-in interview on November 30, 2025, in Vapi – exciting pharma jobs in India!
Contents
About the Company
Indoco Remedies Limited is a fully integrated, research-oriented pharmaceutical company headquartered in Mumbai. With a strong global footprint across 55+ countries and approvals from prestigious regulators like USFDA and UKMHRA, Indoco excels in formulations and APIs.
The company operates state-of-the-art manufacturing facilities and focuses on innovation, quality compliance, and sustainable growth. Known for its dynamic environment, Indoco offers rewarding pharmaceutical careers in India with emphasis on regulatory excellence and employee development.
Job Details
- Company Name: Indoco Remedies Limited (Indoco Analytical Solutions – Stability Centre)
- Experience: 3–12 years (varies by role)
- Qualification: B.Sc./M.Sc. (Chemistry), B.Pharm/M.Pharm
- Location: B-19, MIDC Waluj, Chhatrapati Sambhajinagar (Aurangabad), Maharashtra – 431136
- Work Type: On-site
Job Description
Indoco Remedies is expanding its Stability Centre at Waluj, Chhatrapati Sambhajinagar, and invites skilled professionals for multiple QC and stability-related positions. These roles focus on analytical testing, stability studies, documentation, and regulatory compliance in a USFDA-approved environment. Ideal for candidates seeking growth-oriented QC jobs in the pharmaceutical industry.
Officer / Sr. Officer / Jr. Executive
- Department: Quality Control – Stability
- Role: Analytical execution for stability samples
- Experience: 3 to 5 years
- Education/Qualification: B.Sc./M.Sc. (Chemistry)/B.Pharm/M.Pharm
Executive / Sr. Executive
- Department: Quality Control – Stability
- Role: Advanced analytical testing and instrumentation
- Experience: 5 to 8 years
- Education/Qualification: B.Sc./M.Sc. (Chemistry)/B.Pharm/M.Pharm
Officer (Documentation)
- Department: Quality Control – Stability Documentation
- Role: Data review and compliance documentation
- Experience: Not specified (relevant experience in documentation required)
- Education/Qualification: B.Sc./M.Sc. (Chemistry)/B.Pharm/M.Pharm
Assistant Manager / Deputy Manager
- Department: Quality Control – Stability
- Role: Team leadership, data trending, and audit handling
- Experience: 8 to 12 years
- Education/Qualification: M.Sc. (Chemistry)/M.Pharm
Officer (Report Preparation)
- Experience: 4 to 6 years
- Education/Qualification: M.Sc. (Chemistry)/M.Pharm
Officer (GLP)
- Department: Quality Control – GLP Compliance
- Role: Stability chamber management and instrument calibration
- Experience: 4 to 6 years
- Education/Qualification: B.Sc./B.Pharm
Skills/Qualifications
- Hands-on experience with HPLC, GC, UV Spectrophotometer, Dissolution Apparatus
- Proficiency in Empower Chromatography Software and Caliber LIMS Software
- Expertise in stability studies, analytical method validation/transfer
- Knowledge of ICH guidelines, GLP, good chromatography practices
- Investigative skills for OOS, data trending, and regulatory audits
- Report preparation and review capabilities
- Team management and SOP drafting (for senior roles)
Key Responsibilities
- Perform stability sample analysis
- Operate HPLC, GC, and dissolution equipment
- Review stability and validation data
- Manage stability chambers and calibrations
- Investigate OOS and deviations
- Prepare stability/method reports
- Handle regulatory audits
- Ensure GLP compliance
- Trend stability data
- Lead teams and draft SOPs
Benefits/Perks
- Competitive salary (no constraint for deserving candidates)
- Dynamic work environment with global exposure
- Ample opportunities for professional growth
- Learning and development programs
- Long-term career stability in a reputed pharma company
- Innovative and compliance-focused culture
How to Apply
Candidates unable to attend can email updated CVs to harshali.jaiswal@indoco.com. For more pharma job opportunities, visit Pharma Recruiter. Don’t miss this chance – apply now and advance your pharmaceutical career in India!
Verified Post
Verification: To confirm the legitimacy of this posting, you can view the original announcement on the Lincoln LinkedIn page.
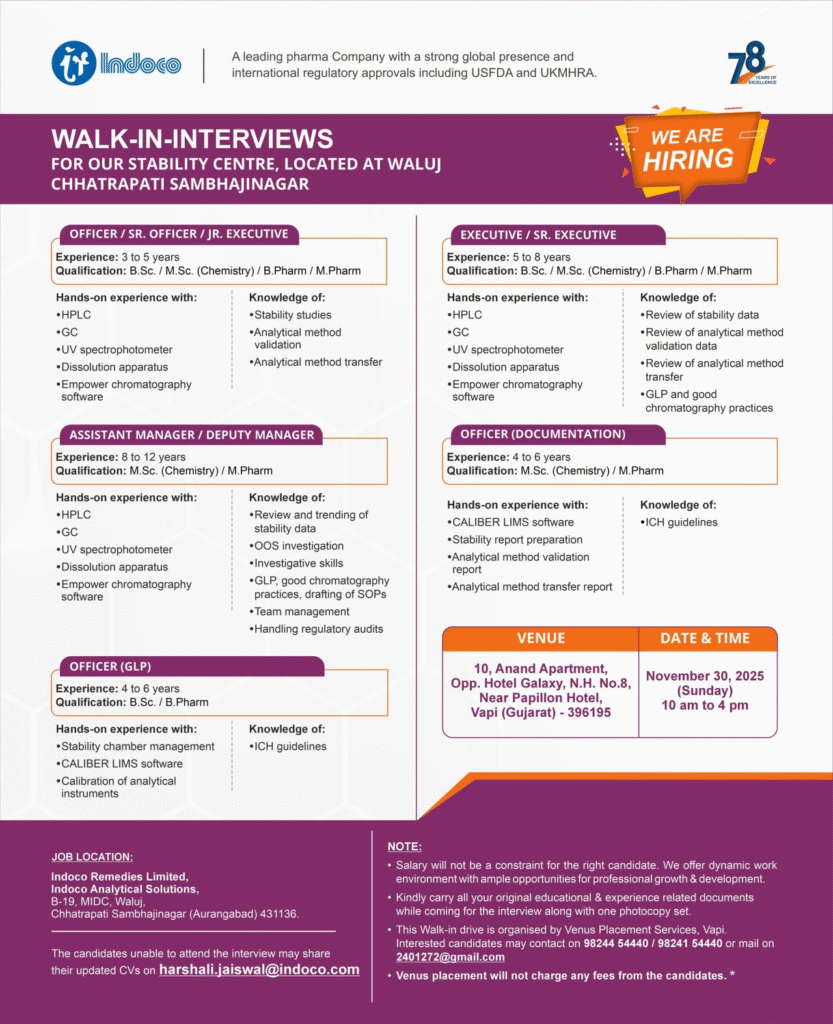
Walk-in Interview Details
- Date: November 30, 2025 (Sunday)
- Time: 10 am to 4 pm
- Venue: 10, Anand Apartment, Opp. Hotel Galaxy, N.H. No.8, Near Papillon Hotel, Vapi (Gujarat) – 396195
- Contact/Email: 98244 54440 / 98241 54440 or 2401272@gmail.com (Organized by Venus Placement Services – no fees charged)
Carry original documents, experience certificates, and one photocopy set.
Why You Should Join
Indoco Remedies offers a vibrant company culture rooted in innovation and regulatory excellence. With USFDA and UKMHRA approvals, it provides long-term career stability and exposure to global standards.
Employees enjoy continuous learning, growth opportunities, and a supportive environment that rewards talent. Join a leader in pharmaceutical careers in India for a fulfilling professional journey.
FAQs
What qualifications are required for these QC jobs at Indoco Remedies?
B.Sc./M.Sc. in Chemistry or B.Pharm/M.Pharm, with 3–12 years of relevant experience in stability/QC.
Is this a walk-in interview or can I apply online?
Both! Attend the walk-in on November 30, 2025, in Vapi or email your CV to harshali.jaiswal@indoco.com.
What documents should I bring to the walk-in interview?
Original educational/experience documents plus one photocopy set.
Are there growth opportunities and good salary packages?
Yes – salary is no constraint for the right candidate, with ample professional growth in a dynamic, global pharma environment.


