Discover top pharma jobs at Innoxel Lifesciences! Attend our walk-in interview on November 16, 2025, for QA jobs, QC jobs, production roles, and pharmaceutical careers in India.
Contents
About the Company
Innoxel Lifesciences Pvt. Ltd. stands as an innovative Contract Development and Manufacturing Organization (CDMO) headquartered in Vadodara, India. Specializing in aseptic and terminal sterilization of injectable vials and ampoules, the company drives excellence in pharmaceutical production.
With a strong focus on regulatory compliance, including a recent successful USFDA inspection, Innoxel fosters growth and global partnerships. Its commitment to quality and cutting-edge technology positions it as a leader in sterile injectables, offering stable careers for pharma professionals.
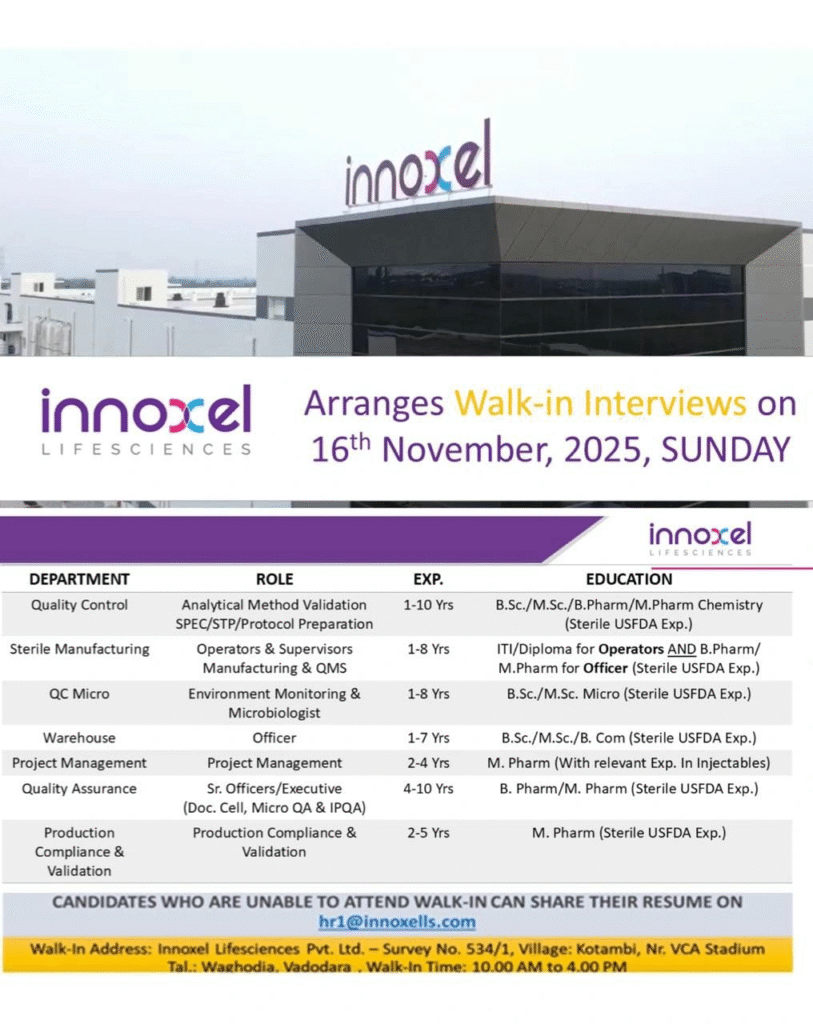
Job Details
- Company Name: Innoxel Lifesciences Pvt. Ltd.
- Experience: Varies by role (1-10 years)
- Qualification: B.Sc./M.Sc./B.Pharm/M.Pharm in Chemistry, Microbiology, or related fields; ITI/Diploma for operators (Sterile USFDA experience required)
- Location: Vadodara, Gujarat, India
- Work Type: On-site
Job Description
Innoxel Lifesciences is expanding its team with urgent openings across key departments like quality control, manufacturing, and assurance. This hiring drive targets skilled professionals in sterile injectables to support USFDA-compliant operations. Multiple roles offer diverse opportunities in pharma jobs, from hands-on production to strategic validation.
Analytical Method Validation SPEC/STP/Protocol Preparation
- Department: Quality Control
- Role: Analytical Method Validation SPEC/STP/Protocol Preparation
- Experience: 1-10 Years
- Education/Qualification: B.Sc./M.Sc./B.Pharm/M.Pharm in Chemistry (Sterile USFDA Exp.)
Operators & Supervisors Manufacturing & QMS
- Department: Sterile Manufacturing
- Role: Operators & Supervisors Manufacturing & QMS
- Experience: 1-8 Years
- Education/Qualification: ITI/Diploma for Operators; B.Pharm/M.Pharm for Officers (Sterile USFDA Exp.)
Environment Monitoring & Microbiologist
- Department: QC Micro
- Role: Environment Monitoring & Microbiologist
- Experience: 1-8 Years
- Education/Qualification: B.Sc./M.Sc. in Microbiology (Sterile USFDA Exp.)
Warehouse Officer
- Department: Warehouse
- Role: Officer
- Experience: 1-7 Years
- Education/Qualification: B.Sc./M.Sc./B.Com (Sterile USFDA Exp.)
Project Management
- Department: Project Management
- Role: Project Management
- Experience: 2-4 Years
- Education/Qualification: M.Pharm (Relevant Exp. in Injectables)
Sr. Officers/Executive (Doc. Cell, Micro QA & IPQA)
- Department: Quality Assurance
- Role: Sr. Officers/Executive (Doc. Cell, Micro QA & IPQA)
- Experience: 4-10 Years
- Education/Qualification: B.Pharm/M.Pharm (Sterile USFDA Exp.)
Production Compliance & Validation
- Department: Production Compliance & Validation
- Role: Production Compliance & Validation
- Experience: 2-5 Years
- Education/Qualification: M.Pharm (Sterile USFDA Exp.)
Skills/Qualifications
- Proficiency in sterile manufacturing and USFDA regulatory standards
- Strong analytical skills for method validation and protocol development
- Knowledge of injectables, microbiology, and quality management systems (QMS)
- Excellent documentation and compliance expertise in QA/IPQA
- Project coordination abilities with a focus on timelines and risk assessment
- Team leadership for supervisors and operators in production environments
- Degree in Pharmacy, Chemistry, or Microbiology with hands-on sterile experience
Key Responsibilities
- Validate analytical methods per USFDA guidelines
- Supervise sterile manufacturing operations daily
- Monitor environmental controls and conduct microbial tests
- Manage warehouse inventory and compliance logs
- Lead injectable project planning and execution
- Review documents for QA in Doc. Cell and IPQA
- Ensure production validation meets regulatory standards
Benefits/Perks
- Robust career growth in a fast-expanding CDMO
- Continuous learning via skill development programs
- Competitive salary packages tailored to experience
- Collaborative work culture emphasizing innovation
- Global exposure through USFDA-compliant projects
How to Apply
To apply for these pharma jobs, candidates unable to attend the walk-in can email their updated resumes to hr1@innoxells.com. Include your role preference and contact details in the subject line.
Verified Post
The post is released by the Innoxel Lifesciences LinkedIn page. Click here to visit the post
For additional pharmaceutical careers in India, explore opportunities at Pharma Recruiter. Seize this chance to elevate your QA jobs or QC jobs journey—apply now and step into a dynamic future at Innoxel!
Walk-in Interview Details
- Date: 16th November 2025, Sunday
- Time: 10:00 AM to 4:00 PM
- Venue: Innoxel Lifesciences Pvt. Ltd., Survey No. 534/1, Village: Kotambi, Nr. VCA Stadium, Tal.: Waghodia, Vadodara, Gujarat
- Contact/Email: hr1@innoxells.com
Why You Should Join
Innoxel Lifesciences cultivates a supportive company culture recognized for skill development, earning a 3.8/5 rating from employees. With long-term career stability in a USFDA-approved facility, you’ll thrive in an environment blending compliance and innovation.
Embrace endless learning opportunities, from advanced sterile techniques to global collaborations, ensuring your pharmaceutical career in India flourishes amid cutting-edge projects and team-driven success.
FAQs
Who is eligible for these pharma jobs at Innoxel?
Candidates with 1-10 years of experience in sterile injectables and USFDA-compliant roles, holding B.Pharm/M.Pharm or relevant science degrees, are ideal. Freshers with strong qualifications may apply for entry-level operator positions.
How can I apply if I can’t attend the walk-in interview?
Simply email your resume to hr1@innoxells.com with the subject “Application for [Role Name] – Innoxel Lifesciences.” We’ll review and schedule virtual interviews promptly.
What should I prepare for the walk-in interview?
Bring your resume, educational certificates, and experience proofs. Expect discussions on sterile manufacturing, QA/QC processes, and USFDA knowledge. The event runs from 10 AM to 4 PM on November 16, 2025.
What are the salary and growth opportunities?
Salaries are competitive, starting from entry-level pharma roles up to executive positions, with performance-based hikes. Innoxel offers rapid promotions, training in injectables, and global exposure for sustained career advancement in production jobs.


