IPCA Laboratories Ltd., a globally recognized pharmaceutical company established in 1949, is seeking talented professionals for its Sikkim Formulation Plant in Rangpo, Sikkim. With over 350 formulations and 80 APIs, IPCA serves 120+ countries and operates USFDA, UK-MHRA, and EDQM-approved facilities. Join our team of over 15,000 professionals to contribute to high-quality oral solid dosage (OSD) manufacturing in a dynamic, employee-friendly environment rated 3.8/5 on AmbitionBox for job security.
Job Details
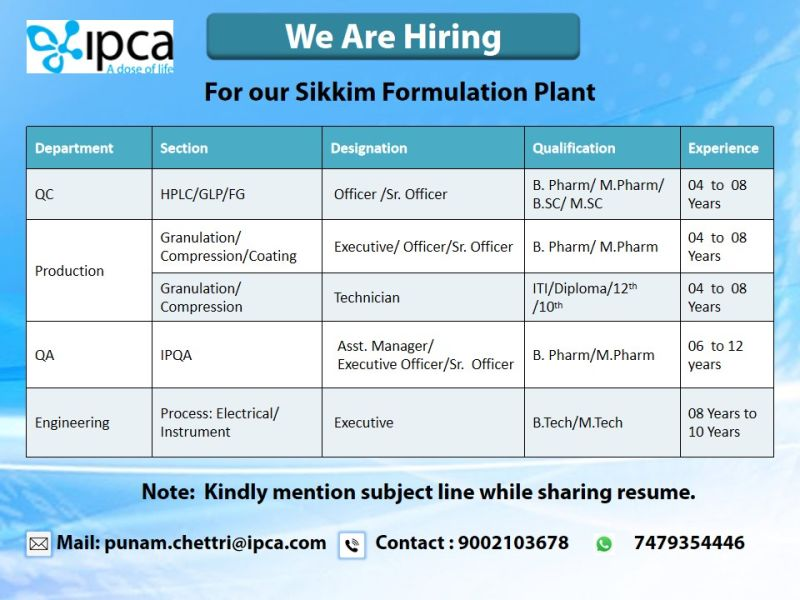
- Job Location: IPCA Laboratories Ltd., Sikkim Formulation Plant, Rangpo, Sikkim
- Application Method: Email resumes to punam.chettri@ipca.com
- Contact: Call +91 9002103678 or +91 7479354446 for inquiries
Required Documents:
- Updated resume
- Educational certificates (B.Pharm/M.Pharm/B.Sc/M.Sc/B.Tech/M.Tech/ITI/Diploma/10th/12th)
- Last 3 months’ payslips and latest increment letter
- Aadhar card and PAN card
- Passport-size photographs (2)
Note:
- Candidates must have experience in regulated plants (e.g., USFDA, UK-MHRA).
- Candidates interviewed at IPCA in the last 6 months are ineligible.
- All positions involve shift duties; male candidates are preferred.
- Mention the specific role and department (e.g., “QC Officer – HPLC” or “Production Executive – Granulation”) in the email subject line.
Open Positions
Quality Control (QC) – HPLC/GLP/FG
- Designation: Officer / Senior Officer
- Qualification: B.Pharm / M.Pharm / B.Sc / M.Sc (Chemistry or related fields)
- Experience: 4–8 years
- Vacancies: Multiple (exact number TBD)
Responsibilities:
- Perform HPLC, GC, and GLP testing for finished goods (FG) and raw materials.
- Conduct method validation, stability studies, and OOS/OOT investigations.
- Maintain LIMS and ensure compliance with cGMP and GLP standards.
- Support regulatory audits (USFDA, MHRA) with accurate documentation.
Key Skills:
- Proficiency in HPLC, GC, and analytical testing.
- Knowledge of SOPs, cGMP, and regulatory guidelines.
Production – Granulation/Compression/Coating
- Designation: Executive / Officer / Senior Officer
- Qualification: B.Pharm / M.Pharm
- Experience: 4–8 years
- Vacancies: Multiple (exact number TBD)
Responsibilities:
- Manage granulation, compression, and coating operations for OSD manufacturing.
- Ensure compliance with SOPs, cGMP, and BMR/BPR documentation.
- Monitor process parameters and support validation activities.
- Coordinate with QA/QC for line clearance and batch release.
Key Skills:
- Hands-on experience in OSD processes and regulatory audits.
- Strong understanding of cGMP and production documentation.
Production – Granulation/Compression
- Designation: Technician
- Qualification: ITI / Diploma / 10th / 12th
- Experience: 4–8 years
- Vacancies: Multiple (exact number TBD)
Responsibilities:
- Operate and maintain granulation and compression equipment.
- Perform line setup, cleaning, and routine checks per SOPs.
- Support production activities and ensure cGMP compliance.
- Assist in batch documentation and equipment troubleshooting.
Key Skills:
- Experience in OSD equipment operation and maintenance.
- Familiarity with cGMP and SOPs.
Quality Assurance (QA) – IPQA
- Designation: Assistant Manager / Executive / Officer / Senior Officer
- Qualification: B.Pharm / M.Pharm / B.Tech / M.Tech
- Experience: 6–12 years
- Vacancies: Multiple (exact number TBD)
Responsibilities:
- Conduct in-process quality checks, line clearance, and sampling for OSD production.
- Review BMR/BPR, manage deviations, CAPA, and change control per QMS.
- Ensure compliance with cGMP, GDP, and regulatory standards (USFDA, MHRA).
- Support media fill studies, validation, and audit preparation.
Key Skills:
- Expertise in IPQA, QMS, and regulatory documentation.
- Strong coordination with production and QC teams.
Engineering – Process: Electrical/Instrument
- Designation: Executive
- Qualification: B.Tech / M.Tech (Electrical or Instrumentation)
- Experience: 8–10 years
- Vacancies: Multiple (exact number TBD)
Responsibilities:
- Perform maintenance and troubleshooting of electrical systems and instrumentation (e.g., PLC, DCS) in OSD manufacturing.
- Conduct preventive maintenance, equipment qualification, and calibration.
- Ensure compliance with cGMP and safety standards during maintenance activities.
- Maintain documentation for regulatory audits (USFDA, MHRA).
Key Skills:
- Expertise in electrical/instrumentation maintenance in regulated plants.
- Knowledge of cGMP, SOPs, and audit requirements.
Why Join IPCA Laboratories?
- Global Leader: Contribute to a company with a ₹2,700 crore turnover, exporting to 120+ countries and manufacturing 350+ formulations and 80+ APIs.
- Regulatory Excellence: Work in a USFDA, UK-MHRA, and EDQM-approved facility, ensuring high-quality standards.
- Employee-Friendly Culture: Rated 3.8/5 on AmbitionBox for job security, with a supportive work environment, though appraisals may be inconsistent (3.2/5).
- Career Growth: Join a dynamic team with opportunities for skill development in a fully integrated pharmaceutical company.
How to Apply
- Submission: Email your updated CV to punam.chettri@ipca.com, mentioning the specific role and department (e.g., “QC Officer – HPLC” or “Production Executive – Granulation”) in the subject line. Include total experience, current CTC, expected CTC, and notice period.
Required Documents:
- Updated resume
- Educational certificates (B.Pharm/M.Pharm/B.Sc/M.Sc/B.Tech/M.Tech/ITI/Diploma/10th/12th)
- Last 3 months’ payslips and latest increment letter
- Aadhar card and PAN card
- Passport-size photographs (2)
Note: Candidates with regulated plant experience (USFDA, MHRA) are preferred.
About IPCA Laboratories
Founded in 1949, IPCA Laboratories Ltd. is a Mumbai-based, fully integrated pharmaceutical company with manufacturing facilities in Sikkim, Ratlam, Athal, and Dewas. Specializing in formulations (OSD, injectables) and APIs, IPCA is a global leader in antimalarials, analgesics, and cardiovascular drugs, with a strong R&D focus (800+ scientists).
Our Sikkim plant is a key OSD facility, known for its high-quality manufacturing and regulatory compliance. Learn more at www.ipca.com.
Important Disclaimer
IPCA Laboratories Ltd. does not charge any fees for recruitment or authorize agencies to collect payments. Report suspicious job offers to hrd@ipca.com.
Join IPCA Laboratories and be a part of delivering “A Dose of Life” through quality healthcare in Sikkim!

