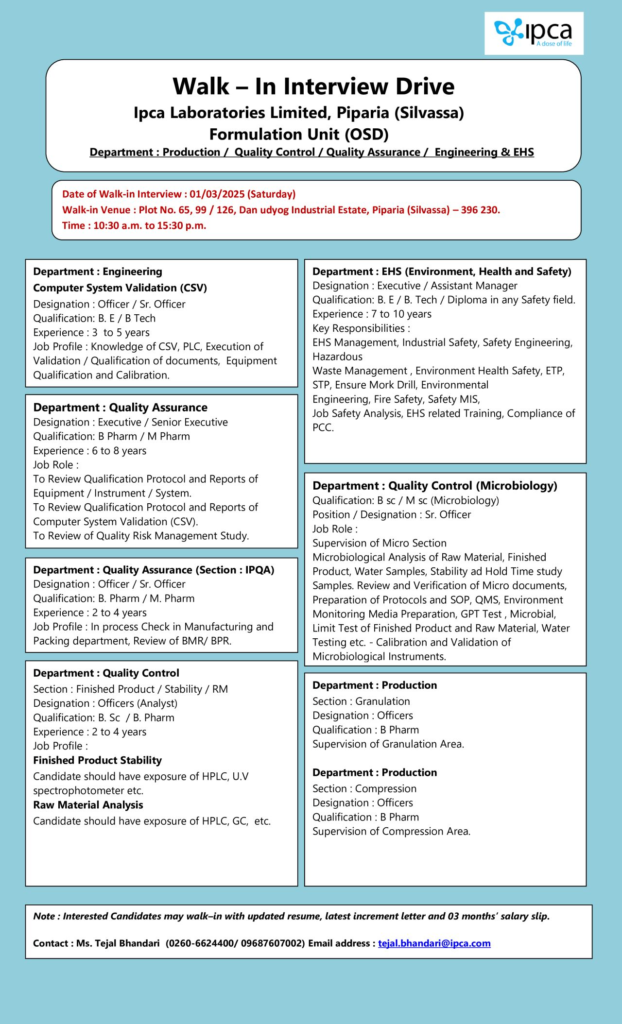
Ipca Laboratories Ltd is conducting a walk-in interview drive for multiple positions across Production, Quality Control, Quality Assurance, Engineering, and EHS departments at its Piparia Silvassa Formulation Unit OSD.
Contents
Interview Details
Date: 1st March 2025 Saturday
Time: 10:30 AM to 3:30 PM
Venue: Plot No. 65, 99/126, Dan Udyog Industrial Estate, Piparia, Silvassa, 396230
Open Positions
Engineering – Computer System Validation CSV
- Designation: Officer or Senior Officer
- Qualification: BE or BTech
- Experience: Three to five years
Key Responsibilities:
- Knowledge of CSV and PLC
- Execution of validation and qualification of documents
- Equipment qualification and calibration
Quality Assurance QA
- Executive or Senior Executive
- Qualification: BPharm or MPharm
- Experience: Six to eight years
Key Responsibilities:
- Review of qualification protocols and reports for equipment, instruments, and systems
- Review of computer system validation CSV
- Review of quality risk management studies
IPQA – Officer or Senior Officer
- Qualification: BPharm or MPharm
- Experience: Two to four years
Key Responsibilities:
- In-process checks in manufacturing and packing departments
- Review of BMR and BPR
Quality Control QC
- Finished Product or Stability or Raw Material – Analyst
- Designation: Officers Analyst
- Qualification: BSc or BPharm
- Experience: Two to four years
Key Responsibilities:
- Finished product and stability testing using HPLC and UV spectrophotometer
- Raw material analysis using HPLC and GC
- Microbiology – Senior Officer
- Qualification: BSc or MSc in Microbiology
Key Responsibilities:
- Microbiological analysis of raw materials, finished products, and water samples
- Stability and hold time study
- Environmental monitoring, media preparation, GPT test, microbial limit testing
- Calibration and validation of microbiological instruments
Environment, Health and Safety EHS
- Designation: Executive or Assistant Manager
- Qualification: BE or BTech or Diploma in Safety
- Experience: Seven to ten years
Key Responsibilities:
- Industrial safety and hazardous waste management
- Fire safety and ETP or STP management
- Conducting mock drills and ensuring safety compliance
Production
- Granulation – Officer
- Qualification: BPharm
- Key Responsibilities: Supervision of granulation area
- Compression – Officer
- Qualification: BPharm
- Key Responsibilities: Supervision of compression area
How to Apply
Interested candidates should walk in with the following documents
- Updated resume
- Latest increment letter
- Last three months salary slips
Contact Person: Ms. Tejal Bhandari
Email: tejal.bhandari@ipca.com
Phone: 0260-6624400 or 9687607002
Do not miss this opportunity to join Ipca Laboratories and grow your career


