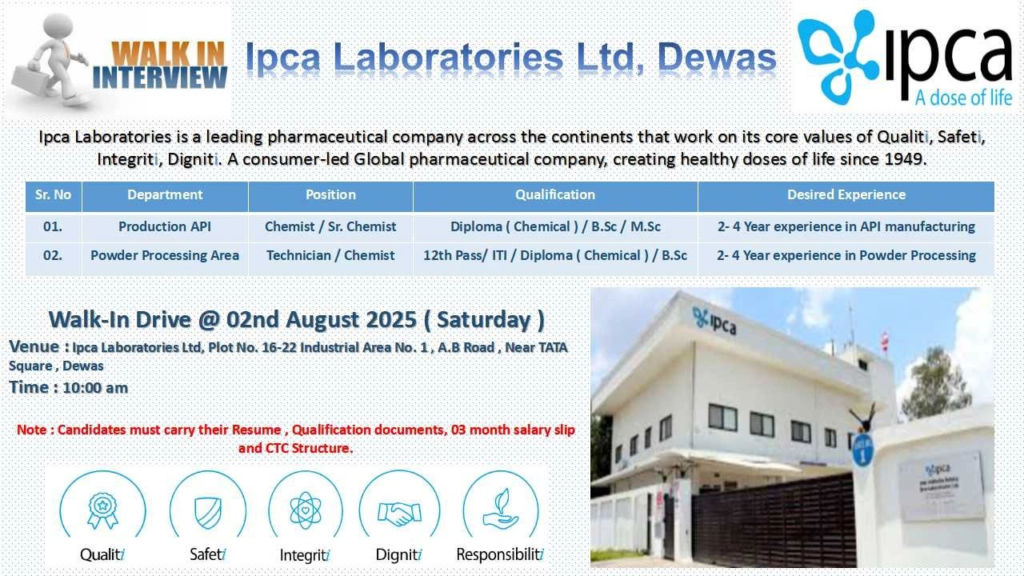
Ipca Laboratories Ltd., a global leader in pharmaceuticals since 1949, is hosting a walk-in interview for its API Manufacturing Unit in Dewas, Madhya Pradesh. Guided by core values of Quality, Safety, Integrity, and Dignity, we invite skilled professionals to join our team.
Contents
About Ipca Laboratories
Ipca Laboratories is a consumer-led pharmaceutical company with a presence in over 110 countries. Our USFDA, UK-MHRA, and WHO-Geneva approved facilities ensure high-quality API production. Learn more at Ipca Laboratories.
Open Positions in Production API
We are hiring for the following roles in our Powder Processing Area at Dewas:
Chemist / Sr. Chemist
- Qualification: Diploma (Chemical) / B.Sc / M.Sc
- Experience: 2-4 years in API manufacturing
- Responsibilities: Oversee powder processing operations, ensure compliance with cGMP, and maintain SOP standards.
Technician / Chemist
- Qualification: 12th Pass / ITI / Diploma (Chemical) / B.Sc
- Experience: 2-4 years in powder processing
- Responsibilities: Handle equipment operations, support API production, and ensure process efficiency.
Walk-In Interview Details
| Detail | Information |
|---|---|
| Date | 2nd August, 2025 (Saturday) |
| Time | 10:00 AM onwards |
| Venue | Ipca Laboratories Ltd, Plot No. 16-22, Industrial Area No. 1, A.B Road, Near TATA Square, Dewas, Madhya Pradesh |
| Contact | Email: hrd.ratlam@ipca.com |
Candidates unable to attend can email their CV to hrd.ratlam@ipca.com.pharmatutor.org
Why Join Ipca Laboratories?
Ipca offers a dynamic work environment with exposure to global regulatory standards (USFDA, MHRA). Our Dewas facility provides opportunities for skill enhancement and career growth in API manufacturing.
Eligibility Criteria
- Relevant qualifications (Diploma, B.Sc, M.Sc, ITI, or 12th Pass).
- 2-4 years of experience in API manufacturing or powder processing.
- Knowledge of cGMP, SOP, and regulatory compliance.
- Commitment to quality and teamwork.
How to Prepare for the Interview
To excel in the interview, candidates should:
- Bring an updated resume and qualification documents.
- Include the last 3 months’ salary slips and CTC structure.
- Be prepared to discuss API manufacturing experience.
- Arrive punctually at the Dewas venue.
Why Dewas?
Dewas is an emerging industrial hub, ideal for pharmaceutical professionals. Ipca’s facility offers cutting-edge technology and a collaborative work culture, fostering professional development.
Application Process
Attend the walk-in interview on 2nd August 2025 with required documents or email your CV to hrd.ratlam@ipca.com. Visit our career page for more opportunities.
Career Growth at Ipca
Ipca prioritizes employee development through hands-on training and exposure to global standards. Join us to build a rewarding career in pharmaceutical manufacturing.
Note
Ipca Laboratories does not charge any fees for job applications. Beware of fraudulent recruitment schemes.
Contact Us
For queries, email hrd.ratlam@ipca.com or visit Ipca Laboratories. Join Ipca and contribute to creating healthy doses of life!
