Ipca Laboratories Limited, a renowned name in the pharmaceutical industry, is hosting a walk-in interview on July 27, 2025, for its Sikkim Formulation Plant. With a strong presence in over 120 countries and approvals from regulatory bodies like USFDA and UK-MHRA, Ipca offers a dynamic environment for professionals passionate about quality healthcare. Visit www.ipca.com to explore our legacy.
Why Ipca Laboratories?
Ipca is a fully integrated pharmaceutical company, manufacturing over 350 formulations and 80 APIs. Our Sikkim facility, focused on oral solid dosage (OSD) formulations, adheres to stringent global standards. Join us to work in a high-quality, employee-friendly environment with opportunities for career growth and skill development.
Open Positions and Requirements
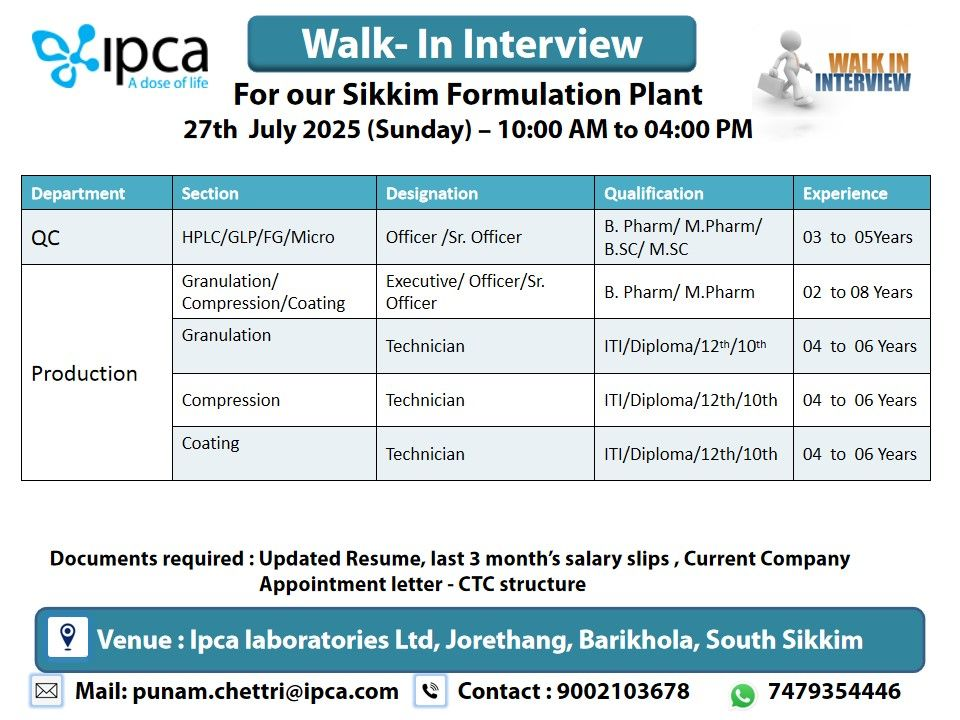
Quality Control (QC) – HPLC/GLP/FG/Micro
- Designation: Officer / Sr. Officer
- Qualification: B.Pharm / M.Pharm / B.Sc / M.Sc
- Experience: 3-5 years
- Skills: Expertise in HPLC, GLP, finished goods (FG) testing, and microbiological analysis.
Production – Granulation/Compression/Coating
- Designation: Executive / Officer / Sr. Officer
- Qualification: B.Pharm / M.Pharm
- Experience: 2-8 years
- Skills: Granulation, compression, coating processes, QMS (CCP, CAPA, Deviation), and GMP documentation.
Production – Granulation
- Designation: Technician
- Qualification: ITI / Diploma / 12th / 10th
- Experience: 4-6 years
- Skills: Hands-on experience in granulation processes and GMP compliance.
Production – Compression
- Designation: Technician
- Qualification: ITI / Diploma / 12th / 10th
- Experience: 4-6 years
- Skills: Proficiency in compression machine operations and troubleshooting.
Production – Coating
- Designation: Technician
- Qualification: ITI / Diploma / 12th / 10th
- Experience: 4-6 years
- Skills: Expertise in coating processes and equipment handling.
Walk-In Interview Details
- Date: Sunday, July 27, 2025
- Time: 10:00 AM to 4:00 PM
- Venue: Ipca Laboratories Ltd, Jorethang, Barikhola, South Sikkim
- Contact Email: punam.chettri@ipca.com
- Contact Numbers: 9002103678, 7479354446
Documents to Bring
Please bring the following:
- Updated resume highlighting relevant experience.
- Last 3 months’ salary slips.
- Current company appointment letter with CTC structure.
- Photocopies of educational certificates, PAN, and Aadhaar card.
Candidate Requirements
We seek candidates with experience in regulated pharmaceutical plants (USFDA/MHRA preferred). Proficiency in HPLC, GLP, granulation, compression, or coating is essential. Candidates must be open to shift duties and demonstrate a strong understanding of cGMP practices.
Why This Opportunity Matters
Ipca’s Sikkim Formulation Plant offers exposure to advanced OSD manufacturing processes and global regulatory standards. With a 4.5/5 rating for career growth, our roles in QC and Production provide a platform to enhance skills and contribute to high-quality pharmaceutical production.
How to Apply
Attend the walk-in interview in Sikkim with all required documents. If unable to attend, email your resume to punam.chettri@ipca.com. Ensure your CV highlights your experience in OSD formulations and regulatory compliance. Ipca does not charge any fees for job applications.
About Ipca Laboratories
Headquartered in Mumbai, Ipca is a global leader in pharmaceuticals, with a turnover exceeding ₹3,120 crores. Our facilities, approved by USFDA and other regulatory bodies, produce high-quality APIs and formulations. Join us to be part of a legacy of innovation and healthcare excellence.
Don’t Miss This Chance!
Mark your calendar for July 27, 2025, and join Ipca Laboratories’ Sikkim team. Whether you’re skilled in quality control or production, we offer a rewarding career path. Attend the walk-in interview and take the next step in your pharmaceutical journey!
