Ipca Laboratories Limited, a globally recognized pharmaceutical company with a legacy of quality, safety, integrity, dignity, and responsibility, is hosting a walk-in interview on Sunday, May 25, 2025, in Indore. With over 75 years of excellence, Ipca is a leading manufacturer of Active Pharmaceutical Ingredients (APIs) and formulations, operating in 110+ countries with approvals from USFDA, UK-MHRA, and other regulatory bodies. Join our dynamic team at our Pologround, Indore facility to contribute to high-quality healthcare solutions!
Contents
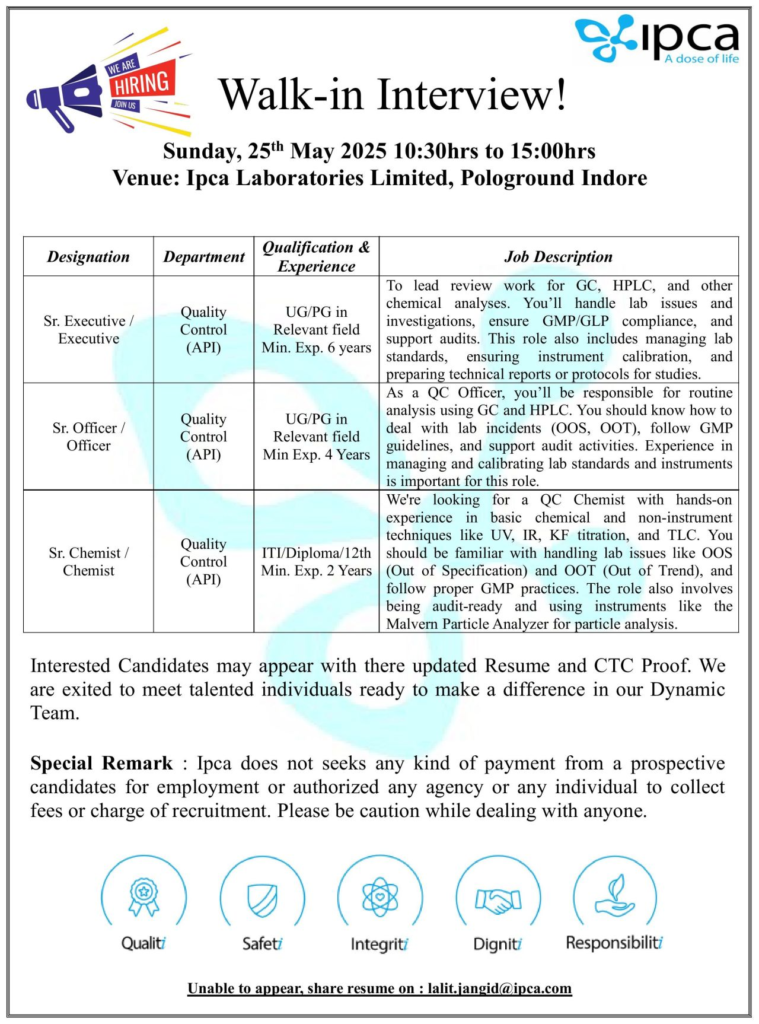
Walk-in Interview Details
- Date: Sunday, May 25, 2025
- Time: 10:30 AM to 3:00 PM
- Venue: Ipca Laboratories Limited, Pologround, Indore, Madhya Pradesh
- Contact Email: lalit.jangid@ipca.com (for those unable to attend)
- Company Website: Ipca Laboratories
Open Positions in Quality Control (API)
1. Senior Executive / Executive – Quality Control (API)
- Qualification: UG/PG in Chemistry or related field
- Experience: Minimum 6 years
- Job Description:
- Lead review work for GC, HPLC, and other chemical analyses.
- Handle lab issues, investigations, and ensure GMP/GLP compliance.
- Support regulatory audits and manage lab standards.
- Ensure instrument calibration and prepare technical reports/protocols for studies.
2. Senior Officer / Officer – Quality Control (API)
- Qualification: UG/PG in Chemistry or related field
- Experience: Minimum 4 years
- Job Description:
- Perform routine analysis using GC and HPLC.
- Address lab incidents (OOS, OOT) and follow GMP guidelines.
- Support audit activities and manage lab standards.
- Calibrate and maintain laboratory instruments.
3. Senior Chemist / Chemist – Quality Control (API)
- Qualification: ITI, Diploma, or 12th
- Experience: Minimum 2 years
- Job Description:
- Conduct basic chemical and non-instrumental analyses (e.g., UV, IR, KF titration, TLC).
- Handle lab issues like OOS and OOT and adhere to GMP practices.
- Maintain audit-readiness and operate instruments like the Malvern Particle Analyzer for particle analysis.
Candidate Requirements
- Documents to Bring:
- Updated resume
- CTC proof (e.g., last 3 months’ salary slips, increment letter)
- Photocopies of educational certificates, Aadhar, and PAN card
- Passport-size photograph
- Note: Candidates unable to attend may email their resume to lalit.jangid@ipca.com.
- Preference: Candidates with experience in API analysis and exposure to USFDA/MHRA audits.
Why Join Ipca Laboratories?
Ipca Laboratories, founded in 1949, is a fully integrated pharmaceutical company with a 4.0/5 rating for job security in Indore, based on employee reviews. Our Indore facility is a hub for API manufacturing, equipped with advanced technology and a supportive work culture rated 3.9/5. We offer opportunities for professional growth in a globally compliant environment. Learn more at Ipca Laboratories.
Important Notice
Ipca Laboratories does not seek payments from candidates for employment or authorize agencies/individuals to collect fees for recruitment. Beware of fraudulent calls or offers.
Join us on May 25, 2025, to advance your career in pharmaceutical quality control jobs in Indore and be part of Ipca’s mission to deliver a dose of life!


