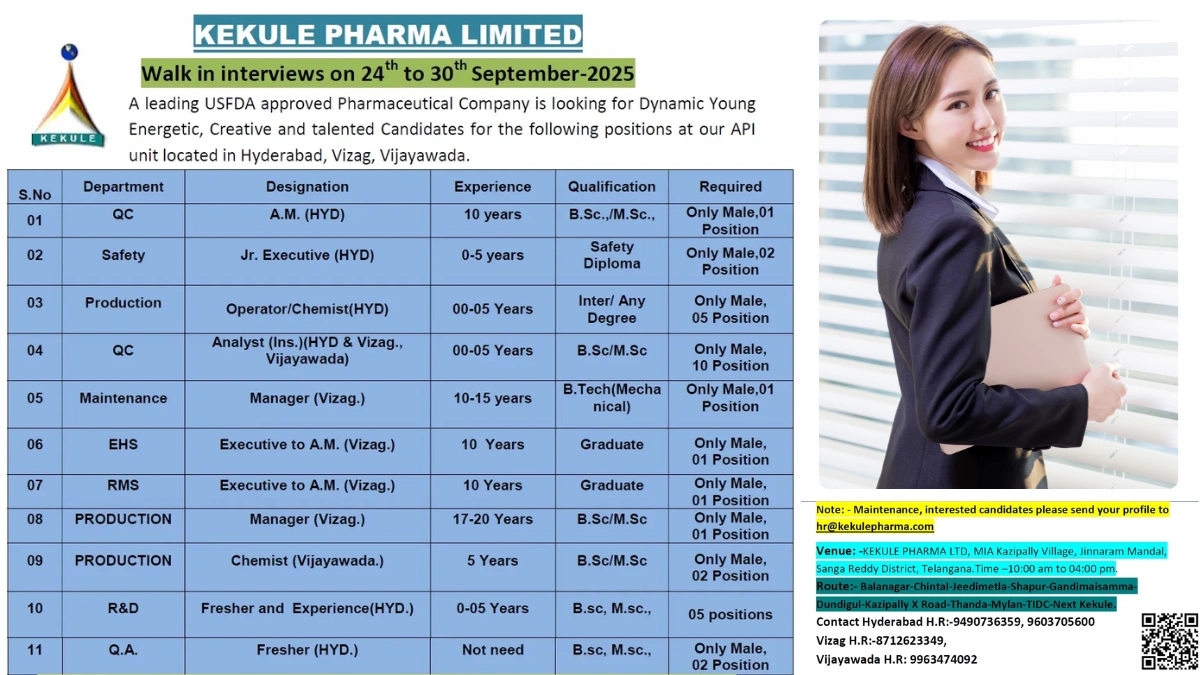
Discover top pharma jobs in India with Kekule Pharma’s walk-in interviews from September 24-30, 2025! Explore QC, Production, and R&D opportunities in API manufacturing.
Contents
About the Company
Kekule Pharma Limited, founded in 1984 by Sridhar Kudaravalli, is a leading Indian pharmaceutical manufacturer based in Hyderabad. Specializing in Active Pharmaceutical Ingredients (APIs) and advanced intermediates, particularly in oncology, the company operates three state-of-the-art facilities accredited by USFDA, MFDS South Korea, COFEPRIS, Iran MOH, and WHO GMP.
With a commitment to innovation, cost efficiencies, and custom synthesis, Kekule exports to over 30 countries and serves global generic players. Regularly audited by multinational corporations, it ensures cGMP compliance and supports CDMO services for complex molecules.
Job Details
- Company Name: Kekule Pharma Limited
- Experience: 0–20 years
- Qualification: B.Sc., M.Sc., B.Tech (Mechanical), Safety Diploma, Graduate, Inter/Any Degree
- Location: Hyderabad, Vizag, Vijayawada (API units)
- Work Type: On-site, Full-time (only male candidates for most positions)
Job Description
Kekule Pharma Limited is hosting walk-in interviews for dynamic candidates to join its USFDA-approved API manufacturing units across Hyderabad, Vizag, and Vijayawada. These pharma jobs focus on quality control, production, maintenance, and R&D, emphasizing oncology APIs and regulatory compliance in a fast-growing environment.
QC A.M.
- Department: QC
- Role: A.M. (HYD)
- Experience: 10 years
- Education/Qualification: B.Sc./M.Sc. (Only Male, 01 Position)
Safety Jr. Executive
- Department: Safety
- Role: Jr. Executive (HYD)
- Experience: 0–5 years
- Education/Qualification: Safety Diploma (Only Male, 02 Positions)
Production Operator/Chemist
- Department: Production
- Role: Operator/Chemist (HYD)
- Experience: 0–5 years
- Education/Qualification: Inter/Any Degree (Only Male, 05 Positions)
QC Analyst (Ins.)
- Department: QC
- Role: Analyst (Ins.) (HYD, Vizag, Vijayawada)
- Experience: 0–5 years
- Education/Qualification: B.Sc./M.Sc. (Only Male, 10 Positions)
Maintenance Manager
- Department: Maintenance
- Role: Manager (Vizag)
- Experience: 10–15 years
- Education/Qualification: B.Tech (Mechanical) (Only Male, 01 Position)
EHS Executive to A.M.
- Department: EHS
- Role: Executive to A.M. (Vizag)
- Experience: 10 years
- Education/Qualification: Graduate (Only Male, 01 Position)
RMS Executive to A.M.
- Department: RMS
- Role: Executive to A.M. (Vizag)
- Experience: 10 years
- Education/Qualification: Graduate (Only Male, 01 Position)
Production Manager
- Department: Production
- Role: Manager (Vizag)
- Experience: 17–20 years
- Education/Qualification: B.Sc./M.Sc. (Only Male, 01 Position)
Production Chemist
- Department: Production
- Role: Chemist (Vijayawada)
- Experience: 5 years
- Education/Qualification: B.Sc./M.Sc. (Only Male, 02 Positions)
R&D Fresher and Experienced
- Department: R&D
- Role: Fresher and Experienced (HYD)
- Experience: 0–5 years
- Education/Qualification: B.Sc./M.Sc. (05 Positions)
Q.A. Fresher
- Department: Q.A.
- Role: Fresher (HYD)
- Experience: Not needed
- Education/Qualification: B.Sc./M.Sc. (Only Male, 02 Positions)
Skills/Qualifications
- Expertise in QC analysis, instrumentation, and compliance for API manufacturing
- Hands-on experience in production operations, synthesis, and oncology intermediates
- Knowledge of EHS, RMS, and maintenance protocols in regulated pharma environments
- Proficiency in R&D for custom synthesis and process development
- Relevant degrees: B.Sc./M.Sc. for technical roles; B.Tech for engineering; Graduate for EHS/RMS
- 0–20 years in USFDA-compliant API facilities, with safety diploma for safety positions
- Strong analytical, problem-solving, and teamwork skills in dynamic settings
- Only male candidates for specified positions; immediate joiners preferred
Key Responsibilities
- Conduct QC testing, instrumentation analysis, and ensure product quality in APIs
- Manage production processes, operator supervision, and synthesis in oncology units
- Oversee maintenance, EHS compliance, and RMS activities for facility safety
- Lead R&D projects for fresher/experienced roles in process innovation
- Handle QA tasks for entry-level positions, including documentation and audits
- Collaborate on regulatory filings, GMP adherence, and team coordination
- Support CDMO services and custom synthesis for global clients
Benefits/Perks
- Competitive salaries in line with API pharma jobs in India
- Career growth from fresher to manager levels in a USFDA-approved leader
- Training in oncology APIs, regulatory compliance, and advanced synthesis
- Inclusive culture fostering innovation and professional development
- Health insurance, on-site facilities, and wellness programs
- Opportunities in export-oriented facilities serving 30+ countries
How to Apply
For maintenance roles, email your profile to hr@kekulepharma.com. For others, attend the walk-in with documents.

For more pharma job resources, visit Pharma Recruiter. Contact HR for queries—apply now!
Walk-in Interview Details
- Date: September 24 to 30, 2025 (Daily)
- Time: 10:00 AM to 4:00 PM
- Venue: Kekule Pharma Ltd, MIA Kazipally Village, Jinnaram Mandal, Sangareddy District, Telangana (Route: Balanagar-Chintal-Jeedimetla-Shapur-Gandimaisamma-Dundigul-Kazipally X Road-Thanda-Mylan-TIDC-Next Kekule)
- Contact/Email: Hyderabad HR: 9490736359, 9603705600; Vizag HR: 8712623349; Vijayawada HR: 9963474092; hr@kekulepharma.com
Why You Should Join
Kekule Pharma Limited offers exceptional pharmaceutical careers in API manufacturing, where your skills in oncology synthesis and quality drive global innovation. In USFDA-approved facilities, thrive in a collaborative, growth-oriented culture with opportunities from entry-level to leadership.
Export to 30+ countries, contribute to life-saving drugs, and enjoy stability in a 40-year legacy of excellence. Join Kekule in Hyderabad, Vizag, or Vijayawada for impactful, rewarding pharma roles.
FAQs
What experience is needed for Kekule Pharma’s API jobs?
Ranges from 0–20 years, with freshers welcome in R&D/QA and seniors for manager roles.
Are only male candidates eligible for these positions?
Yes, most positions specify only male candidates; R&D is open to all.
What documents to bring to the walk-in?
Resume, educational certificates, experience letters, ID proof, and relevant qualifications.