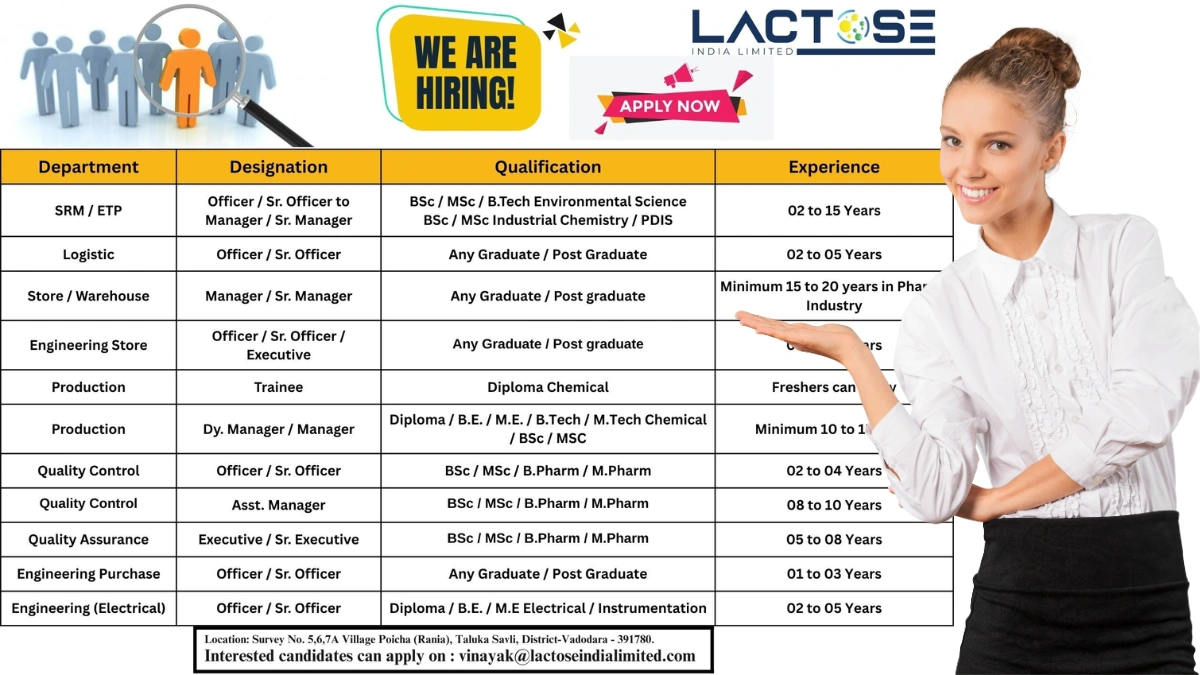
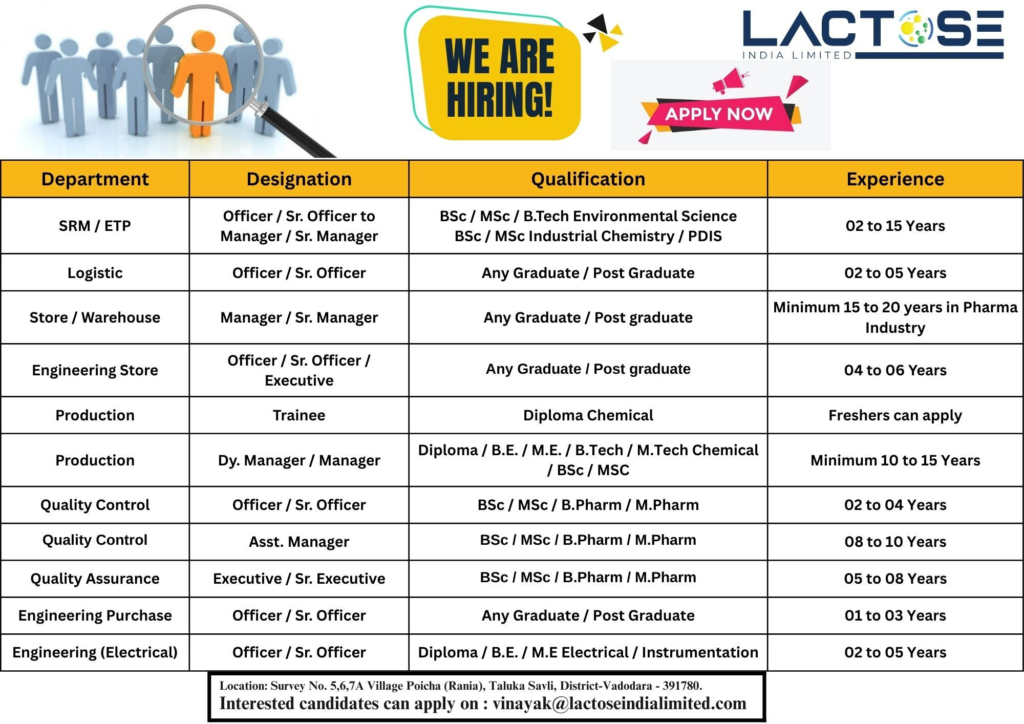
Lactose India Limited, a leading pharmaceutical excipient manufacturer with USFDA and EU-GMP certifications, is hiring for multiple roles at its state-of-the-art facility in Poicha (Rania), Savli, Vadodara, Gujarat. Known for its high-quality lactose monohydrate and contract manufacturing services, Lactose India is rated 3.8/5 for work culture (based on industry reviews) and serves global markets with a ₹100 Cr+ turnover.
Contents
- 1 Job Details
- 2 Job Opportunities
- 2.1 1. SRM/ETP – Officer / Sr. Officer to Manager / Sr. Manager
- 2.2 2. Logistic – Officer / Sr. Officer
- 2.3 3. Store / Warehouse – Manager / Sr. Manager
- 2.4 4. Engineering Store – Officer / Sr. Officer / Executive
- 2.5 5. Production – Trainee
- 2.6 6. Production – Dy. Manager / Manager
- 2.7 7. Quality Control – Officer / Sr. Officer
- 2.8 8. Quality Assurance – Asst. Manager
- 2.9 9. Quality Assurance – Executive / Sr. Executive
- 2.10 10. Engineering Purchase – Officer / Sr. Officer
- 2.11 11. Engineering (Electrical) – Officer / Sr. Officer
- 3 Why Join Lactose India?
- 4 How to Apply
- 5 Important Note
- 6 About Lactose India
- 7 Join Our Mission
Job Details
- Work Location: Lactose India Limited, Survey No. 5, 6, 7A, Village Poicha (Rania), Taluka Savli, District Vadodara – 391780, Gujarat
- Application Email: vinayak@lactoseindialimited.com
- Application Deadline: Apply by May 20, 2025, for priority consideration (inferred based on urgency).
Job Opportunities
1. SRM/ETP – Officer / Sr. Officer to Manager / Sr. Manager
- Qualification: B.Sc. / M.Sc. / B.Tech (Environmental Science), B.Sc. / M.Sc. (Industrial Chemistry) / PDIS
- Experience: 2–15 years
- Salary: ₹3.5–12.0 Lakhs/year (estimated, Vadodara)
- Responsibilities:
- Manage Sewage Treatment Plant (STP) and Effluent Treatment Plant (ETP) operations.
- Ensure compliance with environmental regulations and cGMP.
- Oversee waste management and pollution control systems.
- Preferences: Experience in pharma ETP/STP and knowledge of GPCB norms.
2. Logistic – Officer / Sr. Officer
- Qualification: Any Graduate / Postgraduate
- Experience: 2–5 years
- Salary: ₹3.0–5.5 Lakhs/year (estimated)
- Responsibilities:
- Coordinate transportation, freight, and logistics for raw materials and finished goods.
- Maintain GDP compliance in supply chain operations.
- Preferences: Experience in pharma logistics and ERP systems.
3. Store / Warehouse – Manager / Sr. Manager
- Qualification: Any Graduate / Postgraduate
- Experience: 15–20 years in the pharma industry
- Salary: ₹10.0–15.0 Lakhs/year (estimated)
- Responsibilities:
- Manage warehouse operations, including raw material and finished goods storage.
- Ensure inventory accuracy and compliance with USFDA and EU-GMP standards.
- Preferences: Expertise in pharma warehouse management and QMS documentation.
4. Engineering Store – Officer / Sr. Officer / Executive
- Qualification: Any Graduate / Postgraduate
- Experience: 4–6 years
- Salary: ₹3.5–6.5 Lakhs/year (estimated)
- Responsibilities:
- Handle procurement and inventory of engineering spare parts.
- Support maintenance teams with timely material availability.
- Preferences: Experience in pharma engineering stores and ERP systems.
5. Production – Trainee
- Qualification: Diploma (Chemical)
- Experience: Freshers can apply
- Salary: ₹1.8–2.5 Lakhs/year (estimated)
- Responsibilities:
- Assist in production processes for lactose manufacturing.
- Learn and adhere to cGMP and SOPs.
- Preferences: Willingness to work in shifts and learn pharma production.
6. Production – Dy. Manager / Manager
- Qualification: Diploma / B.E. / M.E. / B.Tech / M.Tech (Chemical) / B.Sc. / M.Sc.
- Experience: 10–15 years
- Salary: ₹8.0–12.0 Lakhs/year (estimated)
- Responsibilities:
- Oversee production of lactose monohydrate and CMO operations.
- Manage BMR/BPR, process optimization, and ICH Q7 compliance.
- Preferences: Experience in USFDA audited plants.
7. Quality Control – Officer / Sr. Officer
- Qualification: B.Sc. / M.Sc. / B.Pharm / M.Pharm
- Experience: 2–4 years
- Salary: ₹3.5–6.0 Lakhs/year (estimated)
- Responsibilities:
- Perform analytical testing (HPLC, GC, UV) for raw materials and finished products.
- Ensure compliance with ICH Q2 and data integrity standards.
- Preferences: Hands-on experience with HPLC and USFDA audits.
8. Quality Assurance – Asst. Manager
- Qualification: B.Sc. / M.Sc. / B.Pharm / M.Pharm
- Experience: 8–10 years
- Salary: ₹6.5–9.0 Lakhs/year (estimated)
- Responsibilities:
- Manage QMS (deviations, CAPA, change control) and batch release.
- Support USFDA/EU-GMP audits and ensure data integrity.
- Preferences: Experience in pharma QA and Trackwise software.
9. Quality Assurance – Executive / Sr. Executive
- Qualification: B.Sc. / M.Sc. / B.Pharm / M.Pharm
- Experience: 5–8 years
- Salary: ₹5.0–7.5 Lakhs/year (estimated)
- Responsibilities:
- Conduct IPQA, line clearance, and QMS documentation.
- Assist in regulatory audits and SOP preparation.
- Preferences: Knowledge of ICH Q9 and GMP compliance.
10. Engineering Purchase – Officer / Sr. Officer
- Qualification: Any Graduate / Postgraduate
- Experience: 1–3 years
- Salary: ₹3.0–5.0 Lakhs/year (estimated)
- Responsibilities:
- Procure engineering materials and negotiate with vendors.
- Maintain purchase records per cGMP standards.
- Preferences: Experience in pharma engineering procurement.
11. Engineering (Electrical) – Officer / Sr. Officer
- Qualification: Diploma / B.E. / M.E. (Electrical / Instrumentation)
- Experience: 2–5 years
- Salary: ₹3.5–6.0 Lakhs/year (estimated)
- Responsibilities:
- Perform maintenance and troubleshooting of electrical/instrumentation systems.
- Support calibration and QMS documentation for engineering.
- Preferences: Knowledge of PLC/SCADA and pharma plant maintenance.
Why Join Lactose India?
- Work in a USFDA and EU-GMP certified facility for pharmaceutical excipients
- Contribute to a niche portfolio of lactose monohydrate and CMO services
- Rated 3.9/5 for growth opportunities (industry reviews)
- Access training in advanced manufacturing and regulatory compliance
- Be part of a 30-year legacy with exports to 20+ countries
How to Apply
Email your CV to vinayak@lactoseindialimited.com with the subject “Poicha – [Designation]” by May 20, 2025. Include:
- Updated resume
- Educational certificates (B.Sc., M.Sc., B.Pharm, M.Pharm, Diploma, B.E., M.E., B.Tech, M.Tech)
- Experience certificates
- Last 3 months’ payslips (if applicable)
- Aadhaar, PAN copies
- Recent passport-size photograph
Preparation Tips
- SRM/ETP: Review ETP/STP operations and GPCB compliance. Be ready for environmental regulation questions.
- Production/QC/QA: Study ICH Q2/Q7/Q9, HPLC, and QMS (CAPA, deviations). Highlight USFDA audit experience.
- Engineering: Prepare for PLC/SCADA, calibration, and electrical troubleshooting. Know cGMP documentation.
- Logistics/Warehouse: Discuss GDP, inventory management, and ERP systems.
- Emphasize regulatory compliance and pharma industry experience.
Important Note
Lactose India Limited does not charge any fees for job applications. Verify communications via @lactoseindialimited.com emails. For queries, contact vinayak@lactoseindialimited.com or call +91-2667-350350 (general contact). Visit www.lactoseindialimited.com for updates.
About Lactose India
Established in 1991, Lactose India Limited, based in Vadodara, is a leading manufacturer of pharmaceutical-grade lactose monohydrate and offers contract manufacturing services. With USFDA, EU-GMP, and ISO 9001:2015 certifications, its Poicha facility supports global clients in the US, EU, and Asia. The company is known for its quality excipients and CMO capabilities, serving major pharma multinationals.
Join Our Mission
Join Lactose India’s mission to deliver high-quality pharmaceutical excipients and CMO services! Apply now for exciting opportunities in Vadodara!