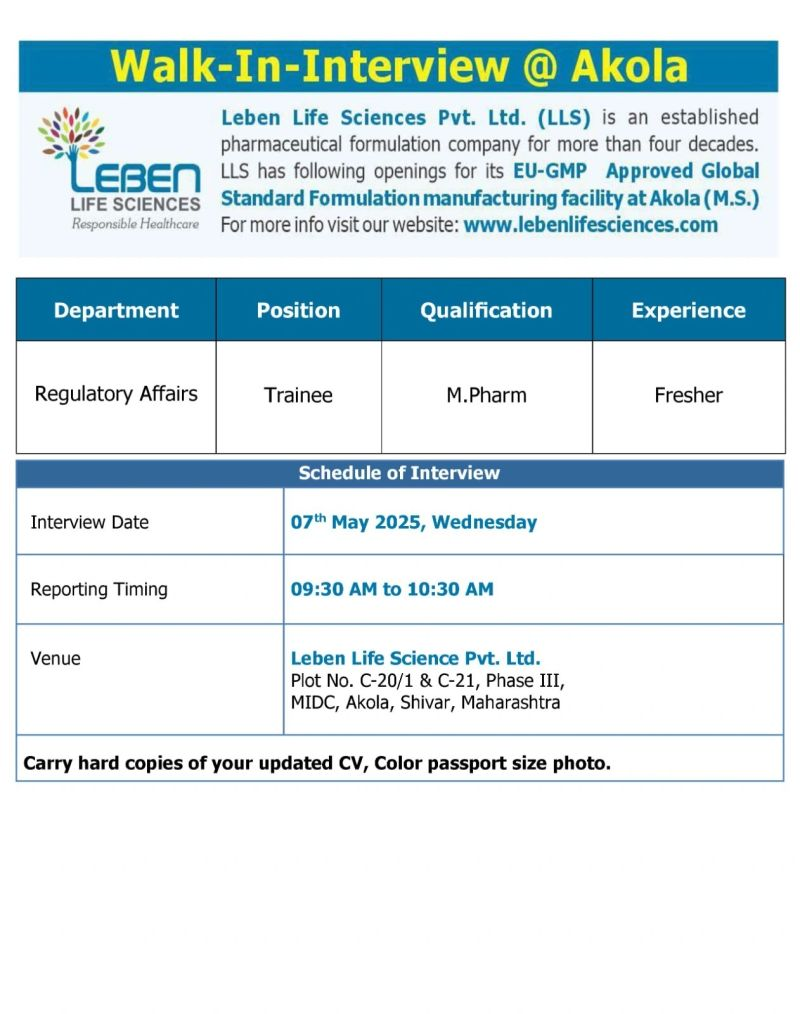
Leben Life Sciences Pvt. Ltd. (LLS), a trusted pharmaceutical formulation company with over four decades of excellence, is hosting a Walk-In Interview for its EU-GMP Approved Global Standard Formulation Manufacturing Facility in Akola, Maharashtra. Committed to Responsible Healthcare, Leben Life Sciences produces high-quality oral solid dosage (OSD) and semi-solid formulations, serving patients in India and 11+ countries.
Join us to kickstart your career in regulatory affairs at our state-of-the-art facility, recognized by WHO-GMP, ISO, and multiple international regulatory bodies.
Contents
Event Details
- Date: Wednesday, May 7, 2025
- Time: 9:30 AM to 10:30 AM
- Venue: Leben Life Sciences Pvt. Ltd., Plot No. C-20/1 & C-21, Phase III, MIDC, Akola, Shivar, Maharashtra
- Contact Email: career@lebenlifesciences.com
- Contact Phone: +91-7498035480
- Website: www.lebenlifesciences.com
Notes:
- Eligibility: Only M.Pharm freshers are eligible for the Regulatory Affairs Trainee position.
- Candidates must bring hard copies of their updated CV, a color passport-size photo, academic certificates, and ID proof.
- Freshers who have attended interviews at Leben Life Sciences in the last 6 months will not be considered.
- Fraud Alert: Leben Life Sciences does not charge fees for recruitment. Verify opportunities via www.lebenlifesciences.com.
Why Join Leben Life Sciences?
Founded in 1981 by Haresh Shah, Leben Life Sciences is one of India’s fastest-growing pharmaceutical companies, with a 3.2/5 work-life balance rating on AmbitionBox based on 70+ employee reviews. Our EU-GMP-certified facility in Akola specializes in tablets, capsules, ointments, and gels, adhering to global standards like USFDA, AFDA-Afghanistan, and ZAMRA-Zambia.
With a legacy of innovation and a focus on affordable, quality medicines, we serve rural and urban communities, curing lakhs of patients annually. Join our team to grow in a supportive environment that values excellence and career development.
Available Job Position: Regulatory Affairs Trainee
We are hiring Regulatory Affairs Trainees to support compliance and documentation for our global formulation manufacturing operations.
Job Details
- Department: Regulatory Affairs
- Position: Trainee
- Qualification: M.Pharm
- Experience: Fresher
- Location: Akola, Maharashtra
Key Responsibilities
- Assist in preparing and reviewing regulatory documentation for product registrations in domestic and international markets
- Support compliance with ICH, CDSCO, and other global regulatory guidelines
- Coordinate with cross-functional teams to compile dossiers and submission packages
- Maintain records of regulatory filings, approvals, and correspondence
- Learn and apply knowledge of GMP and regulatory requirements for OSD and semi-solid formulations
- Participate in training sessions to understand market-specific regulations (e.g., Europe, Africa)
Required Skills and Qualifications
| Category | Details |
|---|---|
| Education | M.Pharm (Pharmaceutics, Pharmaceutical Chemistry, or related specialization) |
| Experience | Fresher (No prior work experience required) |
| Technical Skills | Basic understanding of regulatory guidelines (ICH, CDSCO, USFDA) |
| Soft Skills | Strong communication, attention to detail, and willingness to learn |
| Preferred Knowledge | Familiarity with pharmaceutical formulations (OSD, semi-solids) |
| Tools | Proficiency in MS Office (Word, Excel, PowerPoint) |
Why This Role?
- Career Launchpad: Gain hands-on experience in regulatory affairs at an EU-GMP-certified facility.
- Learning Opportunity: Work under experienced professionals to master global regulatory standards.
- Global Exposure: Contribute to compliance for products exported to 11+ countries.
- Supportive Environment: Join a company with a 53% positive rating for career growth opportunities.
How to Prepare for the Walk-In Interview
To excel at the interview, follow these tips:
- Bring Essential Documents: Hard copies of your updated CV, a color passport-size photo, M.Pharm certificates, and ID proof (Aadhaar/PAN).
- Dress Professionally: Reflect your enthusiasm and commitment to the role.
- Research Leben Life Sciences: Visit www.lebenlifesciences.com to understand our EU-GMP facility, product portfolio, and regulatory certifications.
- Prepare for Questions: Be ready to discuss basic regulatory concepts, GMP principles, or your academic projects in pharmaceutics.
- Arrive Early: Reach the venue by 9:30 AM to complete registration formalities.
Why Akola, Maharashtra?
Akola, an emerging pharmaceutical hub in Maharashtra, offers a vibrant industrial ecosystem and a cost-effective lifestyle. Leben Life Sciences’ facility in MIDC Phase III, Shivar, is equipped with advanced technology, making it an ideal workplace for aspiring regulatory professionals. Learn more about Akola’s industrial growth.
Contact Information
For inquiries or to apply, reach out to:
- Email: career@lebenlifesciences.com (Subject: “Regulatory Affairs Trainee – Walk-In May 7, 2025”)
- Phone: +91-7498035480
- Venue Address: Leben Life Sciences Pvt. Ltd., Plot No. C-20/1 & C-21, Phase III, MIDC, Akola, Shivar, Maharashtra
- Website: www.lebenlifesciences.com
- LinkedIn: Leben Life Sciences Pvt. Ltd.
Don’t miss this opportunity to launch your career with a leading pharmaceutical company. Mark your calendar for May 7, 2025, and join Leben Life Sciences in delivering Responsible Healthcare to the world!


