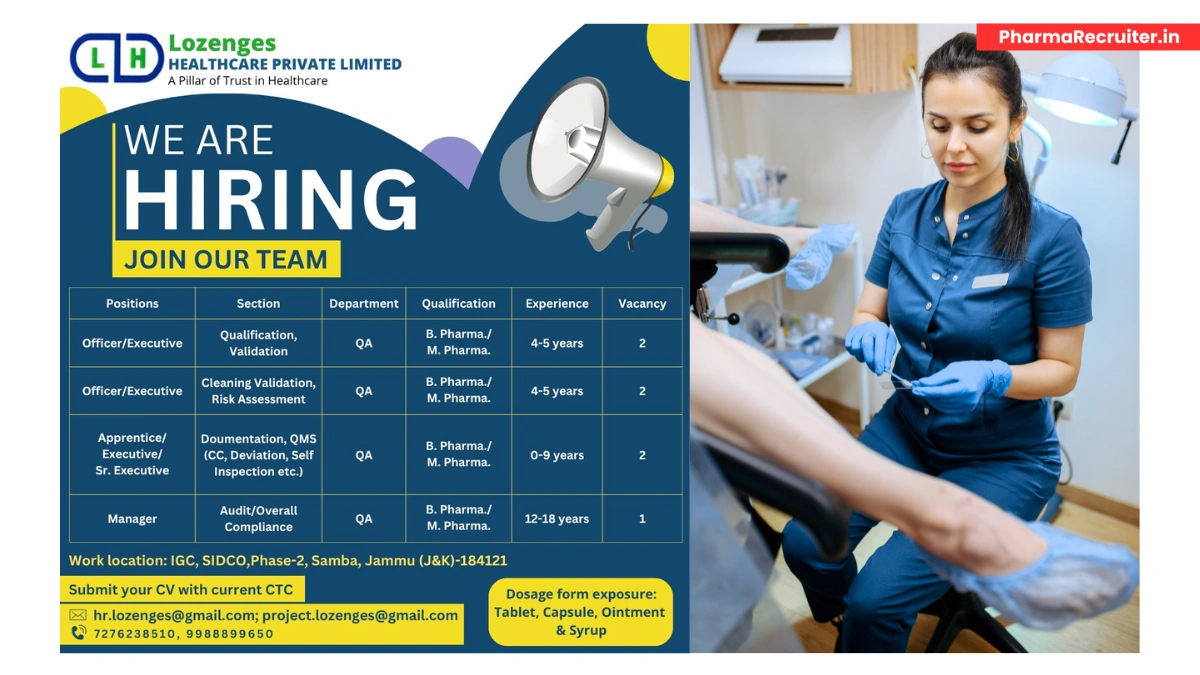
Lozenges LH Healthcare Pvt. Ltd., a trusted name in pharmaceutical manufacturing, is seeking talented professionals for Quality Assurance (QA) roles at our cGMP-compliant facility in Samba, Jammu & Kashmir.
Located in the Industrial Growth Centre (IGC), SIDCO, Phase-2, Samba, J&K – 184121, we specialize in oral solid dosage (tablets, capsules), ointments, and syrups. Join our team to contribute to high-quality healthcare solutions in a region emerging as a pharma hub.
Contents
Why Lozenges LH Healthcare?
Our Samba facility is designed for regulatory compliance, supporting a range of dosage forms with a focus on quality and innovation. While specific employee reviews for Lozenges LH Healthcare are limited, the company operates in Jammu’s growing industrial sector, rated 3.5/5 for job opportunities on LinkedIn.
Salaries for QA roles in similar firms range from ₹3–10 Lakhs/year, with apprentices earning ₹1–2 Lakhs/year. India’s pharmaceutical industry, projected to reach $130 billion by 2030 (Invest India), offers immense growth potential, and our facility provides exposure to diverse dosage forms and regulatory standards.
Job Details
Location: IGC, SIDCO, Phase-2, Samba, Jammu & Kashmir – 184121
Type: Full-time
Dosage Forms: Tablets, Capsules, Ointments, Syrups
Note: Candidates must not have interviewed with Lozenges in the last 6 months. Immediate joiners preferred.
Open Positions
Officer/Executive – Qualification & Validation (QA)
- Qualification: B.Pharm / M.Pharm
- Experience: 4-5 years
- Vacancies: 2
Responsibilities:
- Conduct equipment qualification (DQ, IQ, OQ, PQ)
- Execute process and cleaning validation for tablets, capsules, ointments, and syrups
- Prepare validation protocols and reports
- Ensure compliance with cGMP and regulatory guidelines
Key Skills:
- Expertise in qualification and validation processes
- Knowledge of cGMP, SOPs, and regulatory audits (e.g., USFDA, WHO)
- Strong documentation and analytical skills
Officer/Executive – Cleaning Validation & Risk Assessment (QA)
- Qualification: B.Pharm / M.Pharm
- Experience: 4-5 years
- Vacancies: 2
Responsibilities:
- Perform cleaning validation for manufacturing equipment
- Conduct risk assessments for QA processes
- Support deviation investigations and CAPA implementation
- Ensure compliance with cGMP standards
Key Skills:
- Proficiency in cleaning validation and risk assessment methodologies
- Familiarity with QMS and regulatory compliance
- Attention to detail and problem-solving abilities
Apprentice/Executive/Sr. Executive – Documentation & QMS (QA)
- Qualification: B.Pharm / M.Pharm
- Experience: 0-9 years (Freshers eligible for Apprentice role)
- Vacancies: 2
Responsibilities:
- Manage QA documentation (BMR, BPR, SOPs)
- Handle QMS activities (Change Control, Deviation, Self-Inspection)
- Support Annual Product Quality Review (APQR) preparation
- Ensure compliance with cGMP and internal audits
Key Skills:
- Strong documentation and organizational skills
- Knowledge of QMS (CC, Deviation, CAPA)
- Basic understanding of cGMP and regulatory requirements
Manager – Audit & Overall Compliance (QA)
- Qualification: B.Pharm / M.Pharm
- Experience: 12-18 years
- Vacancies: 1
Responsibilities:
- Oversee QA audits (internal, external, regulatory)
- Ensure overall cGMP compliance across all dosage forms
- Lead QMS implementation and training programs
- Manage regulatory inspections and compliance reports
Key Skills:
- Extensive experience in QA audits and regulatory compliance (USFDA, WHO)
- Leadership in QMS and cross-functional coordination
- Expertise in handling regulatory inspections
How to Apply
- Email: Send your CV with current CTC to hr.lozenges@gmail.com or project.lozenges@gmail.com with subject line “QA [Position] – Samba”
- Documents: Include:
- Updated CV
- Educational certificates (B.Pharm/M.Pharm mark sheets and degree)
- Experience letters, last three months’ salary slips, CTC details
- Aadhaar/PAN card copies
- Contact: HR Team at +91-7276238510 or +91-9988899650

Note: Apply within 7 days. Lozenges LH Healthcare does not charge recruitment fees. Use only official email addresses for communication. Report suspicious activity to HR.
Why a Career with Lozenges LH Healthcare?
Located in Samba, a growing industrial hub with 210+ job opportunities (LinkedIn), our facility offers hands-on experience in QA for diverse dosage forms. Despite the GSTIN cancellation noted in 2022, Lozenges remains active in hiring, reflecting operational continuity.
This role provides exposure to cGMP-compliant processes and regulatory audits, positioning you for growth in India’s thriving pharma sector.
Contact Us
Email hr.lozenges@gmail.com or call +91-7276238510 for details. Join Lozenges LH Healthcare to drive quality excellence in pharmaceuticals!