Lupin Limited, a leading global pharmaceutical company established in 1968, is hosting walk-in interviews for roles at our Tarapur, Maharashtra manufacturing plant. With a presence in 100+ countries and a focus on generics, APIs, and specialty drugs, Lupin operates USFDA, UK-MHRA, and WHO-GMP compliant facilities.
Rated 4.0/5 on AmbitionBox for job security, we invite skilled professionals to join our team of 20,000+ employees to drive excellence in pharmaceutical manufacturing.
Contents
Walk-In Interview Details
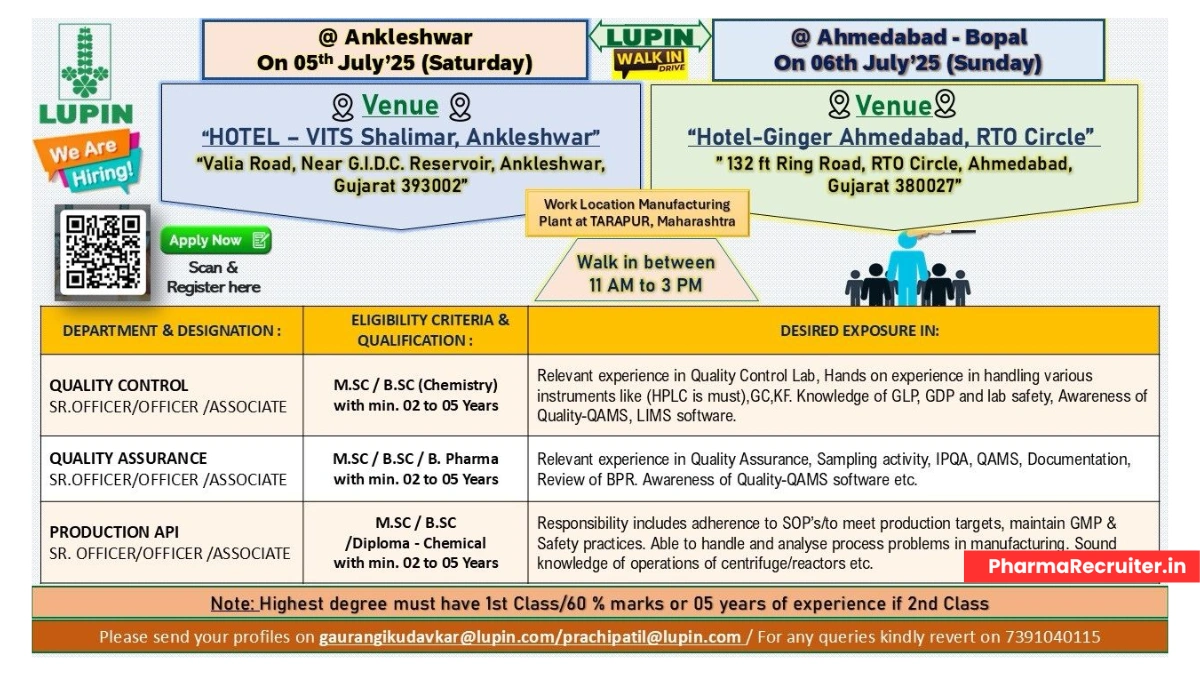
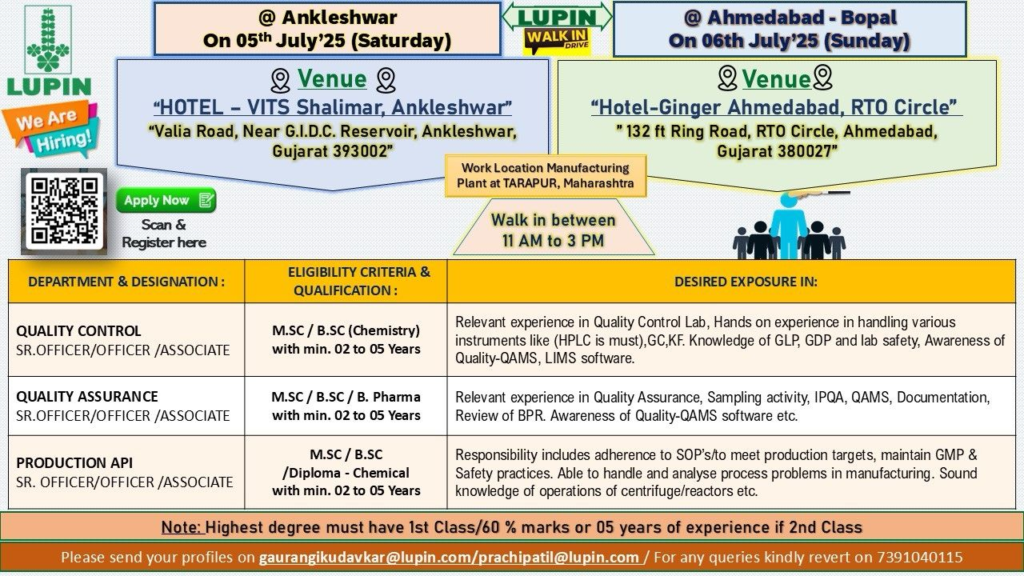
Dates and Venues:
- Ankleshwar: Saturday, July 5, 2025
- Venue: HOTEL – VITS Shalimar, Valia Road, Near G.I.D.C. Reservoir, Ankleshwar, Gujarat – 393002
- Ahmedabad (Bopal): Sunday, July 6, 2025
- Venue: Hotel Ginger Ahmedabad, RTO Circle, 132 ft Ring Road, Ahmedabad, Gujarat – 380027
- Time: 11:00 AM to 3:00 PM
- Work Location: Lupin Limited, Manufacturing Plant, MIDC, Tarapur, Boisar, Maharashtra – 401506
- Pre-Registration: Scan the QR code provided in the original job posting to register.
Contact:
- Email resumes to gaurangikudavkar@lupin.com or prachipatil@lupin.com
- For inquiries, call +91 7391040115
Required Documents:
- Updated resume (2 copies)
- Original and photocopies of educational certificates (B.Sc/M.Sc/B.Pharm/Diploma)
- Last 3 months’ payslips and latest increment letter
- Aadhar card and PAN card
- Passport-size photographs (2)
Note:
- Candidates must have first-class marks (60% or above) in their highest degree or 5+ years of experience if second-class.
- Candidates interviewed at Lupin in the last 6 months are ineligible.
- Experience in regulated plants (USFDA, MHRA) is preferred.
- Immediate joiners preferred.
Open Positions
Quality Control (QC)
- Designation: Senior Officer / Officer / Associate
- Qualification: B.Sc / M.Sc (Chemistry)
- Experience: 2–5 years in Quality Control (API or formulations)
- Vacancies: Multiple (exact number TBD)
Responsibilities:
- Perform analytical testing using HPLC (mandatory), GC, and KF Titrator.
- Ensure compliance with GLP, GDP, and lab safety standards.
- Utilize QAMS and LIMS software for data management.
- Support method validation, stability studies, and regulatory audits (USFDA, MHRA).
Key Skills:
- Hands-on experience with HPLC, GC, and KF Titrator.
- Knowledge of cGMP, GLP, and regulatory documentation.
Quality Assurance (QA)
- Designation: Senior Officer / Officer / Associate
- Qualification: B.Sc / M.Sc / B.Pharm
- Experience: 2–5 years in Quality Assurance (API or formulations)
- Vacancies: Multiple (exact number TBD)
Responsibilities:
- Conduct IPQA and sampling activities for API manufacturing.
- Review Batch Production Records (BPR) and ensure cGMP compliance.
- Manage QAMS, deviations, CAPA, and change control.
- Support regulatory audits and documentation.
Key Skills:
- Expertise in IPQA, QMS, and regulatory compliance (USFDA, MHRA).
- Strong documentation and coordination skills.
Production (API)
- Designation: Senior Officer / Officer / Associate
- Qualification: B.Sc / M.Sc (Chemistry) / Diploma in Chemical Engineering
- Experience: 2–5 years in API production
- Vacancies: Multiple (exact number TBD)
Responsibilities:
- Operate equipment like reactors, centrifuges, and filters to meet production targets.
- Adhere to SOPs, cGMP, and safety practices.
- Analyze and troubleshoot process issues in manufacturing.
- Maintain accurate production records for audits.
Key Skills:
- Hands-on experience with API manufacturing equipment.
- Knowledge of cGMP, safety protocols, and regulatory requirements.
Why Join Lupin Limited?
- Global Leader: Contribute to a company with a $2.7 billion market cap, serving 100+ countries with APIs and formulations in anti-infectives, cardiovascular, and CNS therapies.
- Regulatory Excellence: Work in a USFDA, UK-MHRA, and WHO-GMP compliant facility, known for 200+ ANDAs and 30+ EDMFs.
- Employee-Centric Culture: Rated 4.0/5 on AmbitionBox for job security and culture, with a focus on employee development, though appraisals may be moderate (3.6/5).
- Innovative Environment: Join a team of 20,000+ professionals driving innovation at Tarapur, a key API manufacturing hub.
How to Apply
- Walk-In: Attend the interview on July 5, 2025 (Ankleshwar) or July 6, 2025 (Ahmedabad) at the respective venues with all required documents. Pre-register using the QR code for faster processing.
- For Those Unable to Attend: Email your updated CV to gaurangikudavkar@lupin.com or prachipatil@lupin.com, mentioning the specific role and department (e.g., “QC Officer” or “Production Associate – API”) in the subject line. Include total experience, current CTC, expected CTC, and notice period.
Note: Candidates with regulated plant experience (USFDA, MHRA) are preferred.
About Lupin Limited
Founded in 1968, Lupin Limited is a Mumbai-based pharmaceutical giant with 11 manufacturing facilities, including Tarapur, which specializes in APIs and formulations. With a strong R&D team of 2,000+ scientists and a global presence, Lupin is a leader in generics and specialty drugs, earning ₹20,000 crore in revenue (FY 2024).
The Tarapur plant is a key hub for API production, adhering to stringent regulatory standards. Learn more at www.lupin.com.
Important Disclaimer
Lupin Limited does not charge any fees for recruitment or authorize agencies to collect payments. Report suspicious job offers to hr@lupin.com.
Join Lupin Limited and contribute to quality healthcare at our Tarapur manufacturing plant!