Explore top pharma jobs in Gujarat at Mepro Pharmaceuticals. Join for QC executive roles, regulatory managers, and HR positions. Experienced professionals in B.Pharm/M.Pharm, apply now for Vadodara opportunities!
Contents
About the Company
Mepro Pharmaceuticals Pvt. Ltd., founded in 1982 and headquartered in Vadodara, Gujarat, is a privately owned leader in pharmaceutical manufacturing with over three decades of expertise. Specializing in tablets, capsules, creams, ointments, and sterile products, it exports to more than 40 countries, partnering with top global firms.
Committed to innovation in novel drug delivery systems and stringent regulatory compliance—including WHO approvals—Mepro drives sustainable growth, ensuring high-quality healthcare solutions worldwide.
Job Details
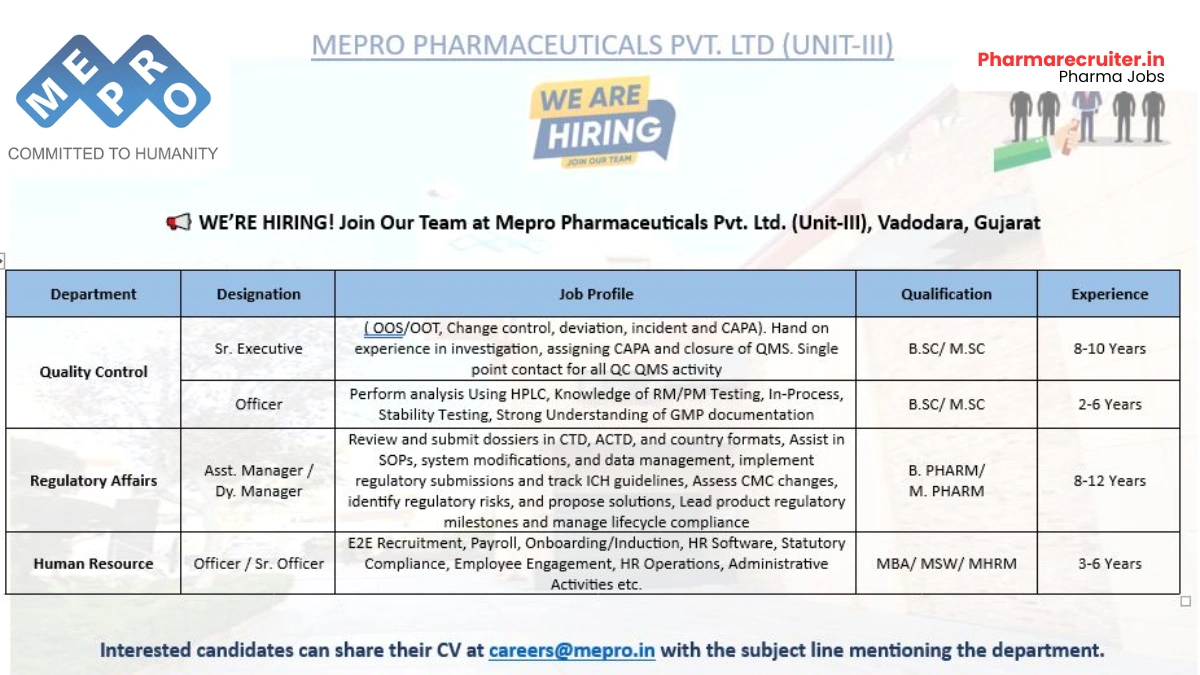
- Company Name: Mepro Pharmaceuticals Pvt. Ltd. (Unit-III)
- Experience: Varies by role (2 to 12 years)
- Qualification: B.Sc./M.Sc. (QC); B.Pharm/M.Pharm (Regulatory Affairs); MBA/MSW/MHRM (HR)
- Location: Vadodara, Gujarat
- Work Type: On-site
Job Description
Mepro Pharmaceuticals Pvt. Ltd. (Unit-III) is expanding its talented team in Vadodara, seeking experts in quality control, regulatory affairs, and human resources to support innovative drug manufacturing.
These roles emphasize GMP compliance, global submissions, and operational excellence, offering a platform to advance pharmaceutical careers in India. Contribute to exporting quality medicines to 40+ countries.
Quality Control – Sr. Executive
- Department: Quality Control
- Market: Global pharmaceutical exports
- Role: Lead OOS/OOT investigations and QMS activities
- Experience: 8 to 10 years
- Education/Qualification: B.Sc./M.Sc.
Quality Control – Officer
- Department: Quality Control
- Market: Global pharmaceutical exports
- Role: Execute analytical testing and documentation
- Experience: 2 to 6 years
- Education/Qualification: B.Sc./M.Sc.
Regulatory Affairs – Asst. Manager/Dy. Manager
- Department: Regulatory Affairs
- Market: Global pharmaceutical exports
- Role: Manage dossier submissions and lifecycle compliance
- Experience: 8 to 12 years
- Education/Qualification: B.Pharm/M.Pharm
Human Resource – Officer/Sr. Officer
- Department: Human Resource
- Market: Global pharmaceutical exports
- Role: Handle recruitment, payroll, and employee engagement
- Experience: 3 to 6 years
- Education/Qualification: MBA/MSW/MHRM
Skills/Qualifications
- Expertise in OOS/OOT, change control, deviations, and CAPA closure for QC
- Proficiency in HPLC analysis, RM/PM testing, and stability studies
- Strong knowledge of CTD/ACTD formats, ICH guidelines, and CMC assessments
- Experience in E2E recruitment, HR software, and statutory compliance
- Excellent GMP documentation and regulatory risk management skills
- Solid communication, problem-solving, and team leadership abilities
Key Responsibilities
- Investigate OOS/OOT trends and assign CAPA actions
- Perform HPLC-based RM/PM and stability testing
- Review dossiers for CTD/ACTD submissions globally
- Lead recruitment drives and onboarding processes
- Track ICH compliance and manage CMC changes
- Ensure payroll accuracy and employee engagement initiatives
Benefits/Perks
- Competitive salary with performance incentives
- Career advancement in a globally exporting firm
- Professional development through training programs
- Collaborative work culture emphasizing innovation
- Health insurance and wellness support
- Opportunities for international regulatory exposure
How to Apply
Interested candidates should email their updated CV to careers@mepro.in, using the subject line “Application for [Department] – [Designation]” (e.g., “Application for Quality Control – Sr. Executive“). Include a brief cover letter highlighting relevant experience.

For tailored advice on QC jobs and regulatory affairs careers, visit Pharma Recruiter. Elevate your pharma career at Mepro—submit your application today!
Why You Should Join
Mepro Pharmaceuticals cultivates a dedicated culture rooted in humanity and excellence, where professionals are recognized for driving quality healthcare innovations. With three decades of stability and expansion into 40+ countries, it promises enduring career growth amid India’s booming pharma sector.
Thrive in a regulatory-compliant environment offering hands-on learning, from CAPA mastery to global dossier expertise, positioning you for leadership in QC roles, HR operations, and beyond.
FAQs
What qualifications are needed for Mepro’s QC positions?
B.Sc./M.Sc. for both Sr. Executive (8-10 years) and Officer (2-6 years) roles, with hands-on HPLC and GMP knowledge essential for pharmaceutical testing in Vadodara.
How does the application process work for regulatory affairs jobs?
Email your CV to careers@mepro.in with the department in the subject. Applications are reviewed promptly; shortlisted candidates contacted for interviews within 1-2 weeks.
Are there HR opportunities for freshers at Mepro?
No, HR roles require 3-6 years experience in recruitment and compliance, but MBA/MSW/MHRM graduates with pharma exposure are ideal for these Gujarat-based positions.
What growth prospects exist in Mepro’s pharma team?
From officer to managerial tracks with global exposure, skill-building in QMS and regulatory submissions, and potential for leadership in an exporting powerhouse.