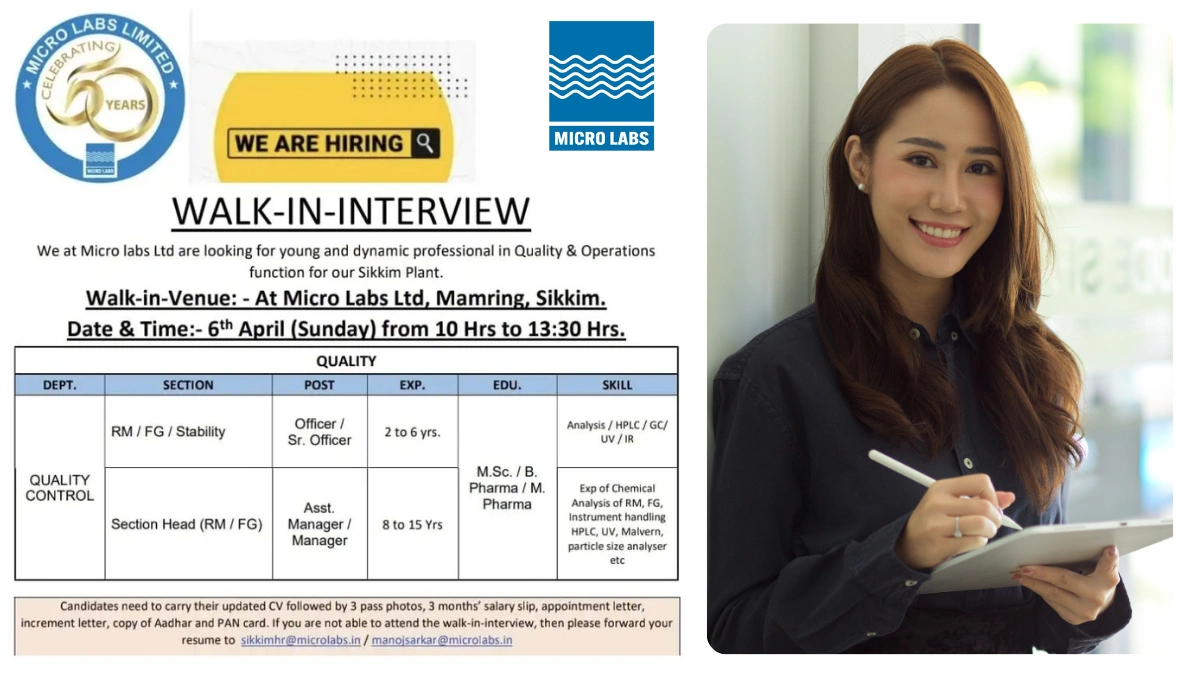
Micro Labs Limited is a dynamic, multi-faceted healthcare organization renowned for its proficient marketing team, state-of-the-art manufacturing facilities, and cutting-edge research and development centers. As we celebrate years of excellence, we are excited to expand our team at our Sikkim Plant by hiring young and talented professionals in Quality and Operations functions. Join us to be part of a leading pharmaceutical company committed to improving lives!
Contents
Walk-In Event Details
Date: 6 April 2025 (Sunday)
Time: 10:00 AM to 1:30 PM
Venue:
Micro Labs Ltd,
Mamring, Sikkim
Job Opportunities
We are seeking skilled professionals for our Quality Control department at the Sikkim Plant. Below are the available positions:
Quality Control – RM/FG/Stability
- Post: Officer / Sr. Officer
- Experience: 2-6 years
- Education: M.Sc / B.Pharm / M.Pharm
Skills:
- Analysis and handling of instruments such as HPLC, GC, UV, and IR.
Quality Control – Section Head (RM/FG)
- Post: Asst. Manager / Manager
- Experience: 8-15 years
- Education: M.Sc / B.Pharm / M.Pharm
Skills:
- Expertise in chemical analysis of raw materials (RM) and finished goods (FG).
- Proficiency in instrument handling, including HPLC, UV, Malvern, and particle size analyzers.
How to Apply
For Walk-In Candidates:
Candidates attending the walk-in interview on 6 April 2025 should bring:
- Updated CV
- 3 passport-size photographs
- Last 3 months’ salary slips
- Appointment letter
- Increment letter
- Copies of Aadhar and PAN cards
For Remote Applicants:
If you are unable to attend the walk-in interview, please forward your resume to:

Why Join Micro Labs?
- Innovative Environment: Work at a state-of-the-art facility in Sikkim with exposure to advanced quality control processes.
- Career Growth: Opportunities to grow within a leading pharmaceutical organization.
- Impactful Work: Contribute to our mission of delivering high-quality healthcare solutions.
Don’t miss this chance to join Micro Labs Limited at our Sikkim Plant! We look forward to meeting you on 6 April 2025 or receiving your application via email. Be a part of our legacy of excellence in quality and operations.