Nectar Lifesciences Ltd (NecLife), a leading name in the pharmaceutical industry, is hosting a walk-in interview for talented professionals to join our Unit 6 facility in Village Bhatoli Kalan, Baddi, Himachal Pradesh. We are hiring for roles across Production (Injection and Oral), HR, Quality Assurance (QA), Quality Control (QC), Store, Engineering, and Microbiology departments. This is an excellent opportunity for candidates with qualifications in B.Pharma, M.Sc., MBA, Diploma, B.Tech, or ITI to contribute to our mission of delivering high-quality pharmaceutical products.
Contents
Event Details
- Date: Monday to Saturday
- Time: 09:00 AM to 05:00 PM
- Venue:
Nectar Lifesciences Ltd, Unit 6
Village Bhatoli Kalan, Adjoining Jhar Majri, Baddi, Himachal Pradesh – 173205, India
View Location on Google Maps
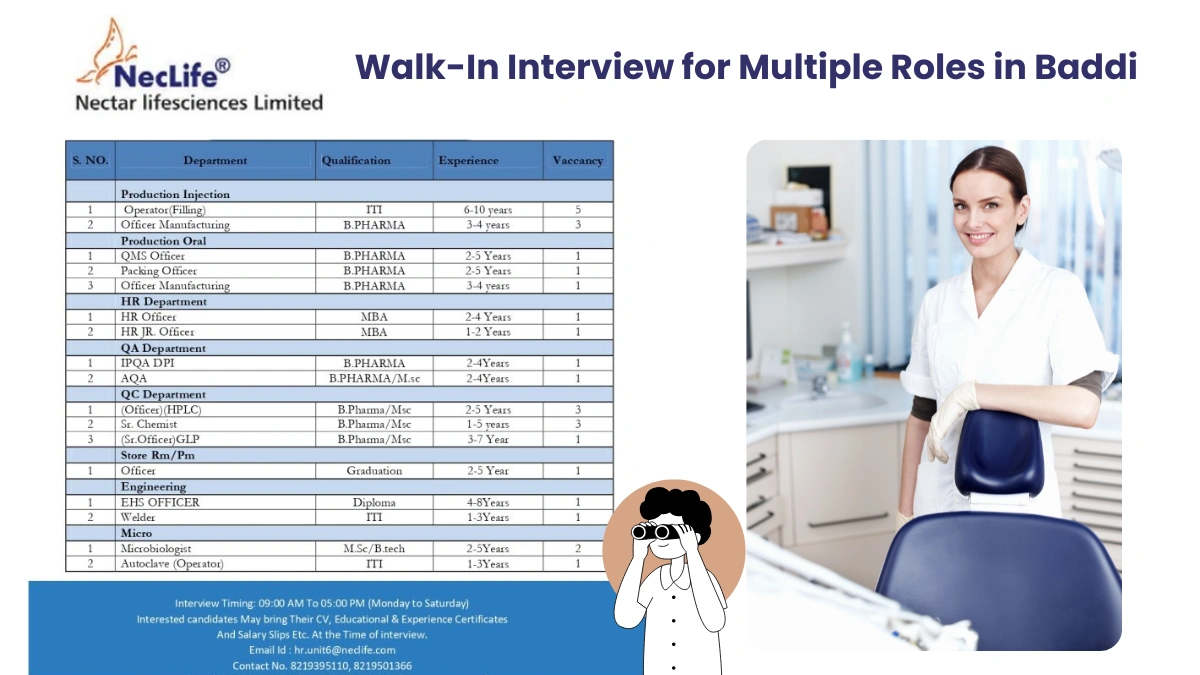
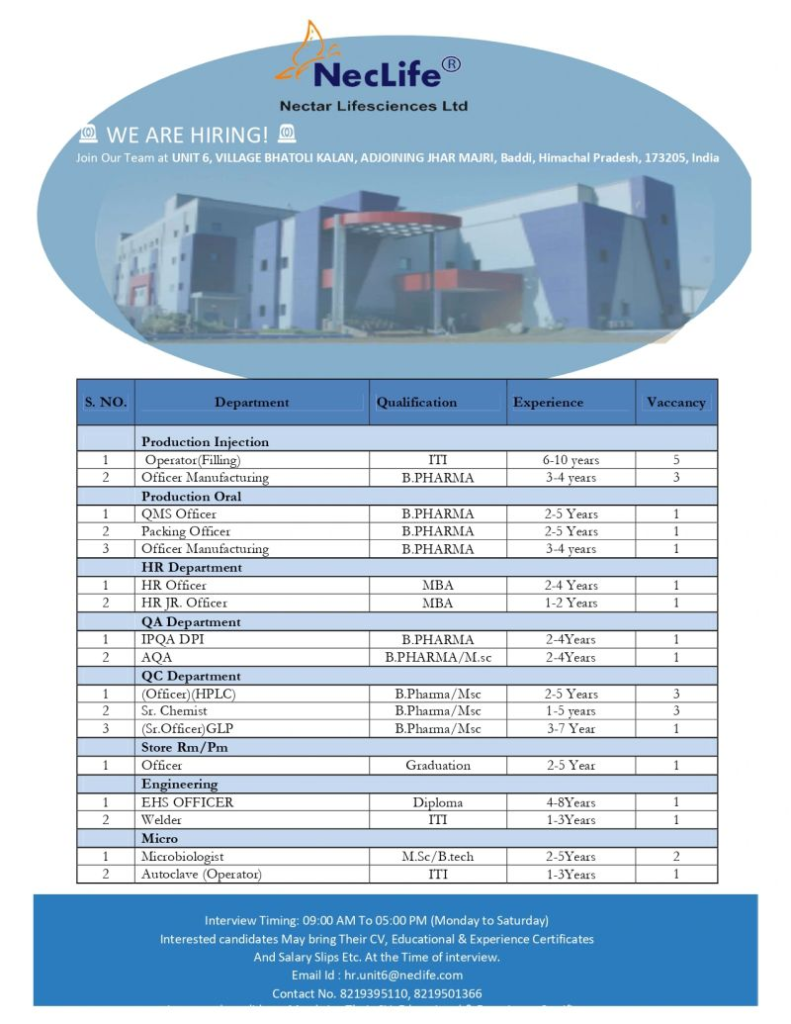
Open Positions and Requirements
We are seeking skilled professionals for various roles in our pharmaceutical manufacturing and support functions. Below are the details of the positions, qualifications, experience, and responsibilities:
Production Injection
Operator (Filling)
- Qualification: Not specified (ITI or relevant technical certification preferred)
- Experience: 6 to 10 years
- Vacancies: 5
- Responsibilities:
- Operate filling machines for injectable products in compliance with Good Manufacturing Practices (GMP).
- Ensure sterility and quality standards during the filling process.
- Perform routine machine maintenance and troubleshooting.
Officer Manufacturing
- Qualification: B.Pharma
- Experience: 3 to 4 years
- Vacancies: 3
- Responsibilities:
- Oversee injectable manufacturing processes, ensuring GMP compliance.
- Coordinate production activities and maintain batch records.
- Monitor process efficiency and implement quality controls.
Production Oral
QMS Officer
- Qualification: B.Pharma
- Experience: 2 to 5 years
- Vacancies: 1
- Responsibilities:
- Manage Quality Management System (QMS) for oral solid dosage (OSD) production.
- Handle deviation, CAPA (Corrective and Preventive Actions), and change control processes.
- Support regulatory audits and ensure GMP compliance.
Packing Officer
- Qualification: B.Pharma
- Experience: 2 to 5 years
- Vacancies: 1
- Responsibilities:
- Manage primary and secondary packing operations for OSD products.
- Ensure track and trace compliance and accurate e-BMR (electronic Batch Manufacturing Record) documentation.
- Maintain GMP standards in packing processes.
Officer Manufacturing
- Qualification: B.Pharma
- Experience: 3 to 4 years
- Vacancies: 1
- Responsibilities:
- Supervise OSD manufacturing processes, including granulation and compression.
- Ensure batch production aligns with GMP guidelines.
- Optimize production efficiency and maintain quality standards.
HR Department
HR Officer
- Qualification: MBA
- Experience: 2 to 4 years
- Vacancies: 1
- Responsibilities:
- Manage recruitment, employee relations, and HR policies.
- Support payroll and compliance with labor regulations.
- Foster a positive workplace culture in a pharmaceutical environment.
HR Junior Officer
- Qualification: MBA
- Experience: 1 to 2 years
- Vacancies: 1
- Responsibilities:
- Assist in HR operations, including onboarding and training.
- Maintain employee records and support HR initiatives.
- Coordinate with senior HR staff to ensure compliance and efficiency.
Quality Assurance (QA) Department
IPQA DPI (In-Process Quality Assurance – Dry Powder Injection)
- Qualification: B.Pharma
- Experience: 2 to 4 years
- Vacancies: 1
- Responsibilities:
- Conduct in-process checks for dry powder injection production.
- Ensure line clearance, sampling, and GMP compliance.
- Support audit readiness and maintain QA documentation.
AQA (Analytical Quality Assurance)
- Qualification: B.Pharma / M.Sc.
- Experience: 2 to 4 years
- Vacancies: 1
- Responsibilities:
- Review analytical data and ensure compliance with regulatory standards.
- Support validation of analytical methods and stability studies.
- Maintain QMS records and assist in regulatory audits.
Quality Control (QC) Department
Officer (HPLC)
- Qualification: B.Pharma / M.Sc.
- Experience: 2 to 5 years
- Vacancies: 3
- Responsibilities:
- Perform HPLC (High-Performance Liquid Chromatography) analysis for raw materials and finished products.
- Ensure quality testing complies with GMP standards.
- Maintain accurate QC documentation and support audit readiness.
Senior Chemist
- Qualification: B.Pharma / M.Sc.
- Experience: 1 to 5 years
- Vacancies: 3
- Responsibilities:
- Conduct chemical analysis of raw materials, intermediates, and finished products.
- Use analytical instruments like UV, FTIR, and GC for testing.
- Ensure compliance with regulatory standards and GMP guidelines.
Senior Officer (GLP – Good Laboratory Practices)
- Qualification: B.Pharma / M.Sc.
- Experience: 3 to 7 years
- Vacancies: 1
- Responsibilities:
- Oversee GLP compliance in QC laboratories.
- Manage instrument calibration and validation processes.
- Support regulatory audits and maintain laboratory records.
Store (Raw Material/Packaging Material)
Officer
- Qualification: Graduation
- Experience: 2 to 5 years
- Vacancies: 1
- Responsibilities:
- Manage raw material and packaging material inventory using SAP/ERP.
- Ensure Good Distribution Practices (GDP) and GMP compliance.
- Coordinate material receipt, storage, and dispatch processes.
Engineering Department
EHS Officer (Environment, Health, and Safety)
- Qualification: Diploma
- Experience: 4 to 8 years
- Vacancies: 1
- Responsibilities:
- Implement EHS policies to ensure a safe working environment.
- Conduct safety audits and risk assessments in the pharmaceutical facility.
- Ensure compliance with environmental regulations and safety standards.
Welder
- Qualification: Not specified (ITI or relevant certification preferred)
- Experience: 1 to 3 years
- Vacancies: 1
- Responsibilities:
- Perform welding tasks for maintenance of production equipment.
- Support mechanical repairs and ensure equipment reliability.
- Adhere to safety protocols during maintenance activities.
Microbiology Department
Microbiologist
- Qualification: M.Sc. / B.Tech
- Experience: 2 to 5 years
- Vacancies: 2
- Responsibilities:
- Conduct microbiological testing for injectable and oral products.
- Ensure sterility and compliance with GMP guidelines.
- Maintain testing records and support audit readiness.
Autoclave Operator
- Qualification: ITI
- Experience: 1 to 3 years
- Vacancies: 1
- Responsibilities:
- Operate autoclave equipment for sterilization processes.
- Ensure sterility standards are met for injectable production.
- Maintain equipment logs and adhere to GMP protocols.
Mandatory Documents to Bring
To ensure a smooth interview process, please bring the following documents:
- Updated resume
- Educational certificates (B.Pharma, M.Sc., MBA, Diploma, B.Tech, ITI)
- Experience certificates (if applicable)
- Last 3 months’ salary slips (if applicable)
- Aadhar card
- One recent passport-size photograph
How to Apply
- In-Person: Attend the walk-in interview at the specified venue with all required documents.
- Outstation Candidates: Email your resume to hr.unit6@neclife.com.
- Contact: For queries, reach out at +91-8219395110 or +91-8219501366.
Why Join Nectar Lifesciences?
- Industry Leader: Be part of a renowned company specializing in injectable and oral pharmaceutical manufacturing.
- Career Growth: Opportunities for professionals to develop skills in production, QA, QC, microbiology, and HR.
- Supportive Environment: Work in a collaborative setting focused on innovation, quality, and regulatory compliance.
- Global Impact: Contribute to high-quality pharmaceutical products that serve international markets.
About Nectar Lifesciences Ltd
Nectar Lifesciences Ltd (NecLife) is a leading pharmaceutical company engaged in the development, manufacturing, and marketing of Active Pharmaceutical Ingredients (APIs), injectables, and oral formulations. Our facility in Baddi, Himachal Pradesh, adheres to global quality standards, delivering innovative healthcare solutions worldwide. Learn more at www.neclife.com.