Discover top pharma jobs in India with Neuland Laboratories’ walk-in interview on September 27, 2025! Explore API production, QA, and QC opportunities at our USFDA-approved Unit-3 in Gaddapotharam. (142 characters)
Contents
About the Company
Neuland Laboratories Limited, established in 1986, is a premier global API Contract Development and Manufacturing Organization (CDMO) headquartered in Hyderabad, India. Specializing in complex active pharmaceutical ingredients for oncology and other therapies, Neuland operates three USFDA-inspected facilities with cGMP compliance from EDQM, PMDA, ANVISA, and more.
Serving over 80 countries with 846+ DMFs filed, the company employs 1,000+ professionals, emphasizing green chemistry, innovation, and sustainability. Neuland’s Unit-3 in Gaddapotharam exemplifies excellence in cleanroom manufacturing and regulatory standards, fostering dynamic pharmaceutical careers.
Job Details
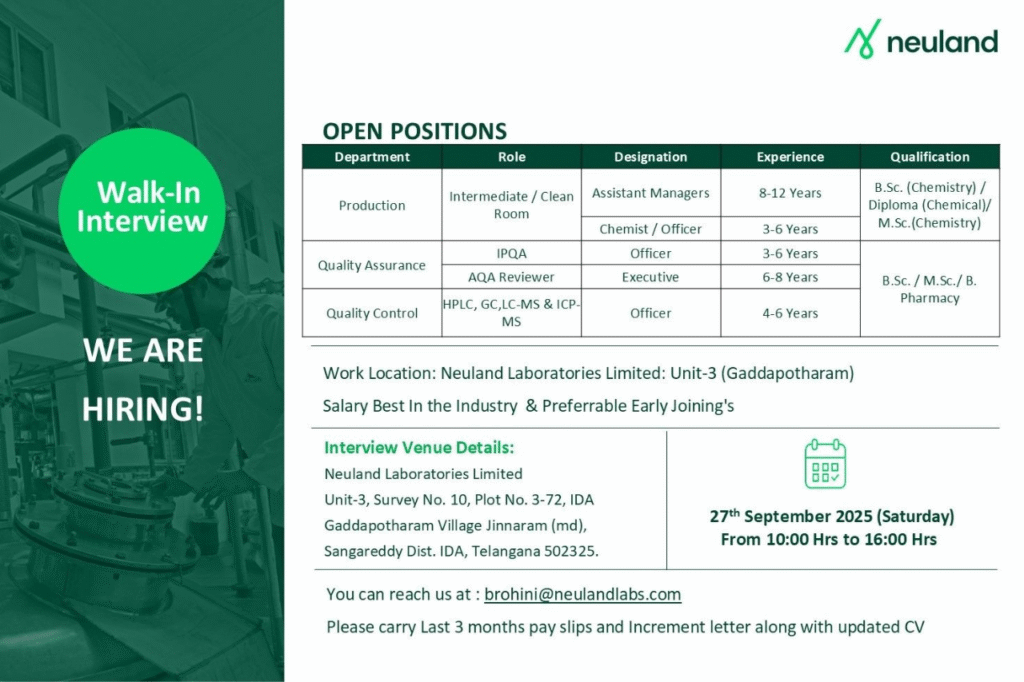
- Company Name: Neuland Laboratories Limited
- Experience: 3–12 years
- Qualification: B.Sc./M.Sc. (Chemistry), Diploma (Chemical), B.Pharmacy
- Location: Unit-3, Survey No. 10, Plot No. 3-72, IDA Gaddapotharam Village, Jinnaram Mandal, Sangareddy Dist., Telangana 502325
- Work Type: On-site, Full-time in API manufacturing
Job Description
Neuland Laboratories is hiring for key roles in production, quality assurance, and quality control at its advanced API Unit-3 in Gaddapotharam. These pharma jobs prioritize expertise in cleanroom operations, IPQA, and analytical instrumentation, supporting global CDMOs in a USFDA-compliant environment.
Production Intermediate / Clean Room
- Department: Production
- Role: Intermediate / Clean Room
- Experience: 8–12 years
- Education/Qualification: B.Sc. (Chemistry) / Diploma (Chemical) / M.Sc. (Chemistry)
Quality Assurance IPQA
- Department: Quality Assurance
- Role: IPQA
- Experience: 3–6 years
- Education/Qualification: B.Sc./M.Sc./B. Pharmacy
AQA Reviewer
- Department: Quality Assurance
- Role: AQA Reviewer
- Experience: 6–8 years
- Education/Qualification: B.Sc./M.Sc./B. Pharmacy
Quality Control HPLC, GC, LC-MS & ICP-MS
- Department: Quality Control
- Role: HPLC, GC, LC-MS & ICP-MS
- Experience: 4–6 years
- Education/Qualification: B.Sc./M.Sc./B. Pharmacy
Skills/Qualifications
- 3–12 years in API production, QA, or QC with hands-on cleanroom and analytical experience
- Proficiency in intermediate synthesis, cleanroom operations, and GMP compliance (Production)
- Expertise in IPQA activities like line clearance, in-process checks, and documentation review
- Strong knowledge of analytical techniques using HPLC, GC, LC-MS, and ICP-MS instruments
- Relevant qualifications: B.Sc./M.Sc. in Chemistry, Chemical Diploma, or B.Pharmacy
- Ability to handle regulatory audits and quality systems in USFDA environments
- Analytical mindset, attention to detail, and teamwork in fast-paced manufacturing
Key Responsibilities
- Execute intermediate and cleanroom production processes per cGMP standards
- Conduct IPQA inspections, sampling, and batch record reviews for compliance
- Review analytical quality assurance documents and ensure data integrity
- Perform testing and analysis using HPLC, GC, LC-MS, and ICP-MS for APIs
- Maintain documentation, adhere to SOPs, and support audit preparations
- Collaborate with cross-functional teams to meet production and quality goals
- Drive continuous improvement in manufacturing and quality processes
Benefits/Perks
- Best-in-industry salaries with preference for early joiners in pharma jobs
- Career advancement in a global CDMO with USFDA and international approvals
- Comprehensive training in green chemistry and advanced API technologies
- Collaborative culture promoting innovation and professional growth
- Health insurance, on-site facilities, and employee wellness programs
- Opportunities to contribute to complex molecules for 80+ countries
How to Apply
Attend the walk-in interview with your updated CV, last 3 months’ payslips, and increment letter. For queries, email brohini@neulandlabs.com.
For more pharma job resources, visit Pharma Recruiter. Apply now to join Neuland’s innovative team!
Walk-in Interview Details
- Date: September 27, 2025 (Saturday)
- Time: 10:00 AM to 4:00 PM
- Venue: Neuland Laboratories Limited, Unit-3, Survey No. 10, Plot No. 3-72, IDA Gaddapotharam Village, Jinnaram Mandal, Sangareddy Dist., IDA, Telangana 502325
- Contact/Email: brohini@neulandlabs.com
Why You Should Join
Neuland Laboratories provides a gateway to exceptional pharmaceutical careers in API development and manufacturing. At our USFDA-inspected Unit-3, leverage cutting-edge cleanrooms and analytical tools to innovate complex therapies for global biotech partners.
With a 38-year legacy of quality and sustainability, enjoy best-in-industry compensation, early joining incentives, and a culture of excellence. Join Neuland in Telangana to drive impactful CDMOs and advance your pharma expertise.
FAQs
What experience is required for Neuland’s pharma jobs?
3–12 years in production, QA, or QC, with specific focus on cleanroom, IPQA, and analytical instruments.
Are early joiners preferred?
Yes, the company offers best-in-industry salaries and prioritizes candidates available for immediate joining.
What documents to bring to the walk-in?
Updated CV, last 3 months’ payslips, increment letter, educational certificates, and ID proof.



I am Interested