The Central Drugs Standard Control Organisation (CDSCO), India’s National Regulatory Authority under the Ministry of Health & Family Welfare, has approved several new drugs in 2025, offering innovative treatment options for various medical conditions.
These approvals include therapies for chronic diseases, infectious diseases, and cancer, addressing critical healthcare needs. Below is a detailed list of new drugs approved by CDSCO in 2025 up to June 21, 2025, including their indications and approval dates.
Contents
List of New Drugs Approved by CDSCO in 2025
The following table summarizes the new drugs approved by CDSCO in 2025, detailing their serial number, name, indication, and date of approval.
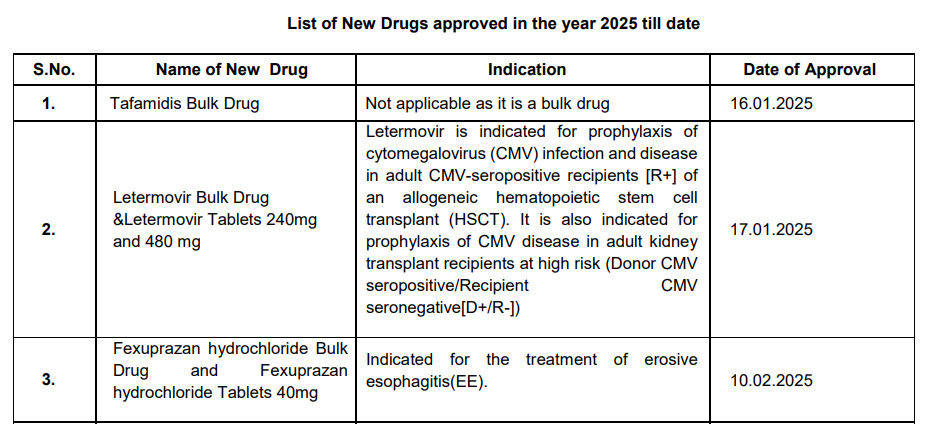
| S.No. | Name of New Drug | Indication | Date of Approval |
|---|---|---|---|
| 1 | Tafamidis Bulk Drug | Not applicable (Bulk Drug) | 16.01.2025 |
| 2 | Letermovir Bulk Drug & Letermovir Tablets (240 mg, 480 mg) | Prophylaxis of cytomegalovirus (CMV) infection and disease in adult CMV-seropositive recipients of allogeneic hematopoietic stem cell transplant (HSCT); prophylaxis of CMV disease in adult kidney transplant recipients at high risk (Donor CMV seropositive/Recipient CMV seronegative) | 17.01.2025 |
| 3 | Fexuprazan Hydrochloride Bulk Drug & Fexuprazan Hydrochloride Tablets (40 mg) | Treatment of erosive esophagitis (EE) | 10.02.2025 |
| 4 | Edoxaban Tosylate Monohydrate Bulk Drug | Not applicable (Bulk Drug) | 11.02.2025 |
| 5 | Edoxaban Tablets (15 mg, 30 mg, 60 mg) | Prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation (NVAF) with risk factors; treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults | 20.02.2025 |
| 6 | Sodium Zirconium Cyclosilicate Powder for Oral Suspension (LOKELMA 5g/10g) | Treatment of hyperkalaemia in adult patients | 05.03.2025 |
| 7 | Rimegepant Oral Disintegrating Tablets (ODT 75 mg) | Acute treatment of migraine with or without aura in adults with previous insufficient response to triptans | 27.03.2025 |
| 8 | Doravirine Bulk Drug & Doravirine Tablets (100 mg) | In combination with other antiretroviral agents for the treatment of HIV-1 infection in treatment-naïve adults and pediatric patients weighing at least 35 kg | 28.03.2025 |
| 9 | Tucatinib Hemiethanolate Bulk Drug & Tucatinib Tablets (50 mg, 150 mg) | In combination with trastuzumab and capecitabine for treatment of adult patients with advanced unresectable or metastatic HER2-positive breast cancer, including those with brain metastases, who have received prior anti-HER2-based regimens | 08.04.2025 |
| 10 | Zanubrutinib Capsules (80 mg) | Treatment of adult patients with mantle cell lymphoma (MCL), Waldenstrom’s macroglobulinemia (WM), relapsed or refractory marginal zone lymphoma (MZL), chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), and relapsed or refractory follicular lymphoma (FL) in combination with obinutuzumab | 08.04.2025 |
| 11 | Linaclotide Bulk Drug & Linaclotide Capsules (72 mcg, 145 mcg) | Treatment of chronic idiopathic constipation in adults | 21.04.2025 |
| 12 | Siponimod Hemifumarate Bulk Drug & Siponimod Tablets (0.25 mg, 1 mg, 2 mg) | Treatment of patients with secondary progressive multiple sclerosis (SPMS) with active disease evidenced by relapses or imaging features of inflammatory activity | 09.05.2025 |
| 13 | Ivosidenib Film-Coated Tablet (250 mg) | In combination with azacitidine for newly diagnosed acute myeloid leukemia (AML) with IDH1 R132 mutation in patients not eligible for standard induction chemotherapy; monotherapy for locally advanced or metastatic cholangiocarcinoma with IDH1 R132 mutation after prior systemic therapy | 14.05.2025 |
| 14 | Tegoprazan Tablet (50 mg) | Treatment of erosive and non-erosive gastroesophageal reflux disease (GERD) and gastric ulcer | 28.05.2025 |
Key Highlights of 2025 Drug Approvals
- Diverse Therapeutic Areas: The approved drugs target a wide range of conditions, including cardiovascular diseases (Edoxaban), neurological disorders (Siponimod, Rimegepant), infectious diseases (Letermovir, Doravirine), and oncology (Tucatinib, Ivosidenib, Zanubrutinib).
- Gastrointestinal Focus: Multiple drugs address gastrointestinal issues, such as Fexuprazan Hydrochloride and Tegoprazan for erosive esophagitis and GERD, and Linaclotide for chronic constipation.
- Bulk Drugs: Several approvals include bulk drugs (e.g., Tafamidis, Edoxaban Tosylate Monohydrate), which are active pharmaceutical ingredients used in drug manufacturing but not directly indicated for treatment.
- Cancer Treatments: Zanubrutinib and Ivosidenib offer advanced options for blood cancers and cholangiocarcinoma, while Tucatinib targets HER2-positive breast cancer, including cases with brain metastases.
Accessing Further Information
For detailed prescribing information and updates on these drugs, visit the official CDSCO website. Additional resources on drug approvals and their implications can be found at:
- U.S. Food and Drug Administration (FDA) for global drug approval insights
- Drugs.com for comprehensive drug information
- Pharmaceutical Technology for industry trends
- Nature Reviews Drug Discovery for scientific perspectives
- BioPharma PEG for insights on blockbuster drugs
Conclusion
The CDSCO’s approval of these 14 new drugs in 2025 reflects India’s commitment to advancing healthcare through innovative therapies. Patients and healthcare providers can explore these new options to address unmet medical needs. Stay updated on further approvals by regularly checking the CDSCO portal or trusted pharmaceutical news sources.
