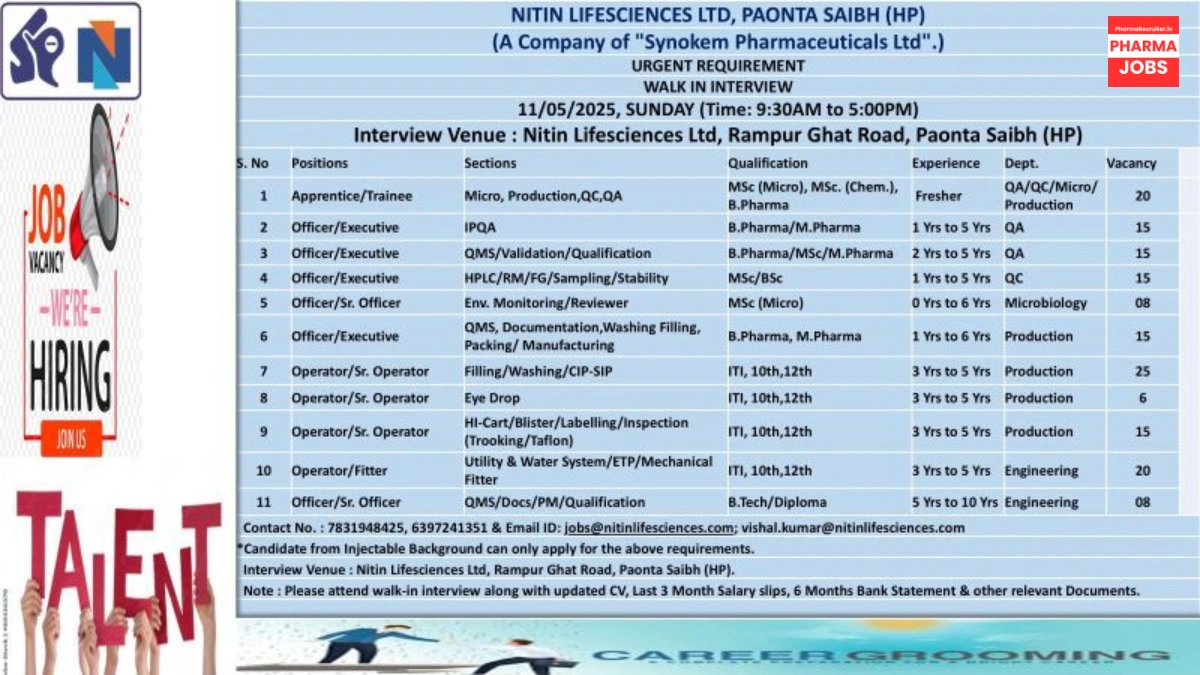
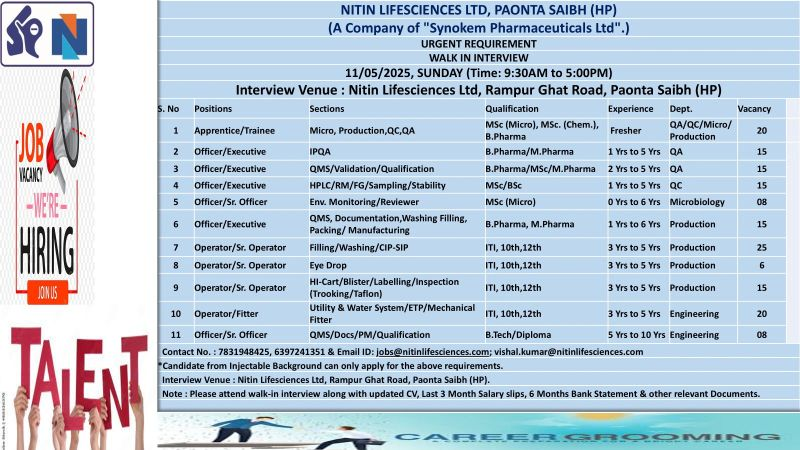
Nitin Lifesciences Ltd., a leading injectable manufacturer and subsidiary of Synokem Pharmaceuticals Ltd., is hosting a Walk-In Interview for multiple positions across Production, Quality Assurance (QA), Quality Control (QC), Microbiology, and Engineering departments on May 11, 2025, at its WHO-GMP-certified facility in Paonta Sahib, Himachal Pradesh.
Established in 1994, Nitin operates three USFDA/EDQM-approved plants, employs 600+ staff, and generates ₹100–500 Crore revenue, exporting to 11+ countries. Rated 3.5/5 for job security on AmbitionBox (150+ reviews), it scores 3.3/5 for work-life balance due to shift demands. Salaries range from ₹2–8 Lakhs per annum (Indeed estimates). Candidates with injectable background only. Join Nitin to drive pharmaceutical excellence
Contents
- 1 Walk-In Interview Details
- 2 Why Join Nitin Lifesciences?
- 3 Job Positions
- 3.1 1. Apprentice/Trainee (Micro, Production, QC, QA)
- 3.2 2. Officer/Executive (IPQA)
- 3.3 3. Officer/Executive (QMS/Validation/Qualification)
- 3.4 4. Officer/Executive (HPLC/RM/FG/Sampling/Stability)
- 3.5 5. Officer/Sr. Officer (Environmental Monitoring/Reviewer)
- 3.6 6. Officer/Executive (QMS, Documentation, Washing, Filling, Packing/Manufacturing)
- 3.7 7. Operator/Sr. Operator (Filling/Washing/CIP-SIP)
- 3.8 8. Operator/Sr. Operator (Eye Drop)
- 3.9 9. Operator/Sr. Operator (Hi-Cart/Blister/Labeling/Inspection)
- 3.10 10. Operator/Fitter (Utility & Water System/ETP/Mechanical Fitter)
- 3.11 11. Officer/Sr. Officer (QMS/Docs/PM/Qualification)
- 4 How to Prepare for the Walk-In
- 5 Why Paonta Sahib, Himachal Pradesh?
- 6 Contact Information
Walk-In Interview Details
- Date: Sunday, May 11, 2025
- Time: 9:30 AM – 5:00 PM
- Venue: Nitin Lifesciences Ltd., Rampur Ghat Road, Paonta Sahib, Dist. Sirmaur, Himachal Pradesh 173025
- Application Method: Attend walk-in with documents or email CV to jobs@nitinlifesciences.com or vishal.kumar@nitinlifesciences.com, subject: “Walk-In [Position] – Paonta Sahib.” WhatsApp CV to +91-7831948425 or +91-6397241351 (no calls).
- Contact: +91-7831948425, +91-6397241351, jobs@nitinlifesciences.com, vishal.kumar@nitinlifesciences.com
- Website: www.nitinlifesciences.com
Notes:
- Fraud Alert: Nitin does not charge fees. Verify via www.nitinlifesciences.com or info@nitinlifesciences.com.
Why Join Nitin Lifesciences?
Nitin’s Paonta Sahib facility, WHO-GMP and ISO 9001:2000 certified, specializes in small volume parenterals (SVPs), dry powder injectables, and eye/ear drops, with products like Pipracillin-Tazobactam and Ceftriaxone-Sulbactam. Acquired by Synokem in 2023 and backed by Recipharm (2016–2023), Nitin supports 200+ formulations for MNCs.
Rated 3.6/5 for learning, it offers robust training but faces feedback on slow appraisals (3.4/5). The roles align with India’s $24.4 billion pharma export market, growing at 10% CAGR (Invest India). Benefits include health insurance and transport allowances.
Job Positions
1. Apprentice/Trainee (Micro, Production, QC, QA)
- Vacancies: 20
- Qualification: M.Sc (Microbiology, Chemistry), B.Pharm
- Experience: Freshers
- Sections: Microbiology, Production, Quality Control, Quality Assurance
- Responsibilities: Assist in injectable production, IPQA, HPLC testing, environmental monitoring; maintain BMR/BPR and GLP.
- Skills: Basic GMP, teamwork, attention to detail.
- Pay: ₹1.8–3 Lakhs/year (Indeed estimates).
- Why This Role?: Ideal for freshers, with 70% transitioning to permanent roles within 2 years (industry trends).
2. Officer/Executive (IPQA)
- Vacancies: 15
- Qualification: B.Pharm, M.Pharm
- Experience: 1–5 years
- Responsibilities: Conduct IPQA for injectable lines, verify BMR/BPR, ensure cGMP compliance, support USFDA audits.
- Skills: IPQA, 21 CFR Part 211, audit preparation, documentation.
- Pay: ₹3–5 Lakhs/year.
- Why This Role?: Lead compliance for SVPs, with 90% of IPQA tasks supporting DMF filings (industry trends).
3. Officer/Executive (QMS/Validation/Qualification)
- Vacancies: 15
- Qualification: M.Sc, B.Sc (Chemistry)
- Experience: 1–5 years
- Responsibilities: Manage QMS (change control, CAPA), validate equipment (IQ/OQ/PQ), prepare SOPs for injectables.
- Skills: ICH Q9/Q10, validation protocols, QMS tools.
- Pay: ₹3–5 Lakhs/year.
- Why This Role?: Enhance QMS expertise, critical for USFDA audits.
4. Officer/Executive (HPLC/RM/FG/Sampling/Stability)
- Vacancies: 15
- Qualification: B.Pharm, M.Sc, M.Pharm
- Experience: 2–5 years
- Responsibilities: Perform HPLC analysis, test RM/FG, conduct stability studies, ensure data integrity.
- Skills: HPLC, Empower, USP <1225>, ICH Q2.
- Pay: ₹3.5–6 Lakhs/year.
- Why This Role?: Master analytical testing, with 80% of tasks linked to regulatory submissions.
5. Officer/Sr. Officer (Environmental Monitoring/Reviewer)
- Vacancies: 8
- Qualification: M.Sc (Microbiology)
- Experience: 0–6 years
- Responsibilities: Conduct environmental monitoring, review microbiological data, ensure sterility for injectables.
- Skills: Aseptic techniques, USP <1116>, audit readiness.
- Pay: ₹3–6 Lakhs/year.
- Why This Role?: Critical for sterile compliance, with high demand in injectable plants.
6. Officer/Executive (QMS, Documentation, Washing, Filling, Packing/Manufacturing)
- Vacancies: 15
- Qualification: B.Pharm, M.Pharm
- Experience: 1–6 years
- Responsibilities: Oversee QMS, document production, manage washing/filling/packing for injectables.
- Skills: GMP, BMR/BPR, aseptic processing.
- Pay: ₹3–6 Lakhs/year.
- Why This Role?: Drive end-to-end production compliance.
7. Operator/Sr. Operator (Filling/Washing/CIP-SIP)
- Vacancies: 25
- Qualification: ITI, 10th, 12th
- Experience: 3–5 years
- Responsibilities: Operate filling/washing/CIP-SIP systems, maintain BMR, ensure aseptic conditions.
- Skills: CIP-SIP, GMP, equipment handling.
- Pay: ₹2–4 Lakhs/year.
- Why This Role?: Hands-on role with 95% batch release contribution.
8. Operator/Sr. Operator (Eye Drop)
- Vacancies: 6
- Qualification: ITI, 10th, 12th
- Experience: 3–5 years
- Responsibilities: Operate eye drop production lines, fill BMR, ensure GMP compliance.
- Skills: Aseptic filling, eye drop manufacturing.
- Pay: ₹2–4 Lakhs/year.
- Why This Role?: Specialized role with growing demand.
9. Operator/Sr. Operator (Hi-Cart/Blister/Labeling/Inspection)
- Vacancies: 15
- Qualification: ITI, 10th, 12th
- Experience: 3–5 years
- Responsibilities: Handle blister packing, labeling, and inspection (Trooking/Teflon), maintain log books.
- Skills: Packing operations, GMP, attention to detail.
- Pay: ₹2–4 Lakhs/year.
- Why This Role?: Ensure quality packaging for exports.
10. Operator/Fitter (Utility & Water System/ETP/Mechanical Fitter)
- Vacancies: 20
- Qualification: ITI, 10th, 12th
- Experience: 3–5 years
- Responsibilities: Maintain water systems, ETP, and mechanical equipment, support injectable plant utilities.
- Skills: Utility maintenance, ETP operations, fitting.
- Pay: ₹2–4 Lakhs/year.
- Why This Role?: Critical for plant operations continuity.
11. Officer/Sr. Officer (QMS/Docs/PM/Qualification)
- Vacancies: 8
- Qualification: B.Tech, Diploma (Mechanical, Electrical)
- Experience: 5–10 years
- Responsibilities: Manage engineering QMS, document PM schedules, qualify utilities for injectables.
- Skills: QMS, PM, equipment qualification.
- Pay: ₹4–8 Lakhs/year.
- Why This Role?: Lead engineering compliance for USFDA standards.
How to Prepare for the Walk-In
- Bring Documents: Updated CV, payslips (last 3 months), bank statements (last 6 months), educational certificates, experience letters, Aadhaar/PAN copies.
- Dress Professionally: Formal attire (e.g., shirt, trousers).
- Interview Prep: Study USFDA 21 CFR Part 211, ICH Q2/Q9, GMP, and injectable processes (e.g., aseptic filling, HPLC). Be ready to discuss experience (e.g., “How did you handle a sterility failure?”). Review Synokem’s acquisition of Nitin (2023). Address appraisal concerns diplomatically, as 35% report delays (AmbitionBox).
- Research Nitin: Visit www.nitinlifesciences.com for injectable portfolio (e.g., Paracetamol Inj., Ceftriaxone) and USFDA compliance. Check Synokem’s role as parent company.
- Arrive Early: Reach by 9:00 AM; expect 1–2 hour wait (AmbitionBox). Transport available from Paonta Sahib bus stand.
Why Paonta Sahib, Himachal Pradesh?
Paonta Sahib, an excise-free pharma hub, hosts Nitin’s USFDA-approved facility, part of Himachal Pradesh’s $2 billion pharma sector (10% CAGR, Invest India). Accessible via NH-7, it offers 300+ injectable jobs (Naukri) and affordable living (₹8,000/month for 1BHK, AmbitionBox). Ideal for sterile manufacturing careers.
Contact Information
- Phone: +91-7831948425, +91-6397241351
- Email: jobs@nitinlifesciences.com, vishal.kumar@nitinlifesciences.com
- Venue/Work Address: Nitin Lifesciences Ltd., Rampur Ghat Road, Paonta Sahib, Dist. Sirmaur, Himachal Pradesh 173025
- Corporate Office: Synokem Pharmaceuticals Ltd., 14/486, DDA Flats, Kalkaji, New Delhi 110019
- Website: www.nitinlifesciences.com
- LinkedIn: Nitin Lifesciences Ltd.
Join Nitin Lifesciences on May 11, 2025, at Paonta Sahib to advance injectable manufacturing. Apply now for career grooming with Synokem!