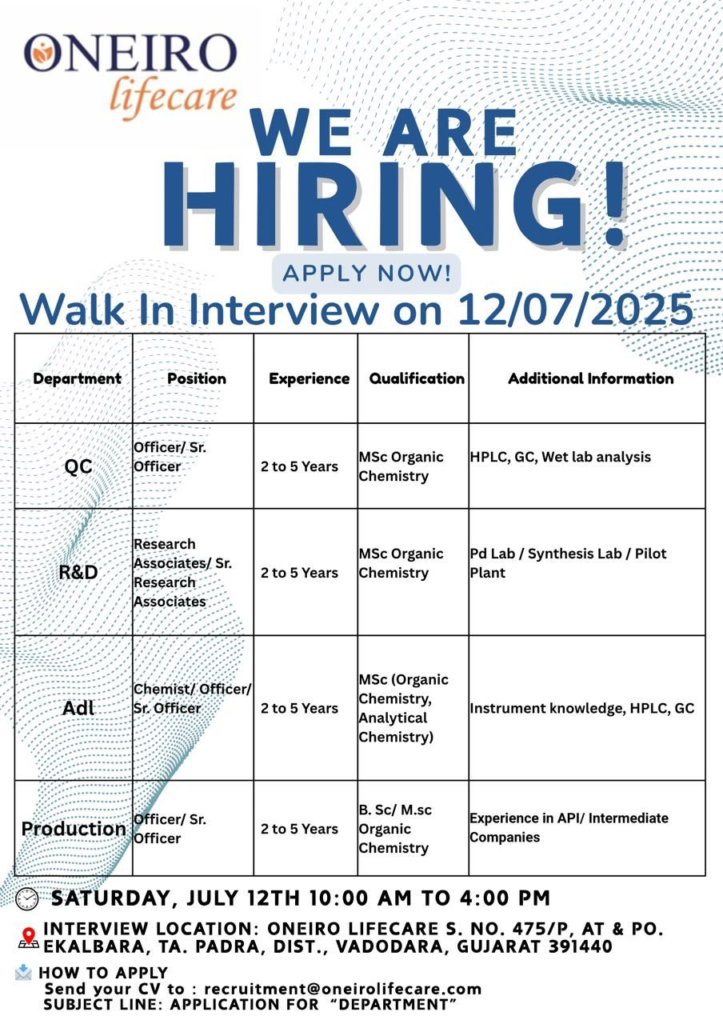
Oneiro Lifecare Pvt. Ltd., a leading ISO 9001:2008 and GMP-certified pharmaceutical manufacturer specializing in APIs, pellets, and intermediates, is hosting a walk-in interview on July 12, 2025, at our facility in Ekalbara, Vadodara, Gujarat.
We invite talented professionals to join our dynamic team in Quality Control (QC), Research & Development (R&D), Analytical Development Laboratory (ADL), and Production departments. Seize this opportunity to advance your career with a globally accredited organization.
Walk-In Interview Details
Date: Saturday, July 12, 2025
Time: 10:00 AM to 4:00 PM
Venue: Oneiro Lifecare, S. No. 475/P, At & Po. Ekalbara, Ta. Padra, Dist. Vadodara, Gujarat – 391440
Contact: Email CVs to recruitment@oneirolifecare.com with the subject line: APPLICATION FOR [DEPARTMENT]
Note: Candidates unable to attend can email their CVs to the above address.
Documents to Bring
- Updated resume
- Last 3 months’ salary slips
- Latest appointment letter
- Passport-size photograph
- Qualification certificates (M.Sc., B.Sc.)
Available Positions
We are hiring for multiple roles across our QC, R&D, ADL, and Production departments at our Vadodara facility. Below are the details:
Quality Control (QC) – Officer / Senior Officer
- Qualifications: M.Sc. in Organic Chemistry
- Experience: 2–5 years
Skills:
- Proficiency in HPLC, GC, and wet lab analysis.
- Knowledge of analytical method validation and stability testing.
- Familiarity with GMP and regulatory compliance (USFDA, EDQM).
Research & Development (R&D) – Research Associate / Senior Research Associate
- Qualifications: M.Sc. in Organic Chemistry
- Experience: 2–5 years
Skills:
- Expertise in synthesis, PD lab operations, and pilot plant activities.
- Experience in multi-step organic synthesis and process development.
- Familiarity with characterization techniques like NMR, LCMS, and HPLC.
Analytical Development Laboratory (ADL) – Chemist / Officer / Senior Officer
- Qualifications: M.Sc. in Organic Chemistry or Analytical Chemistry
- Experience: 2–5 years
Skills:
- Hands-on experience with HPLC, GC, and other analytical instruments.
- Knowledge of method development, validation, and equivalency protocols.
- Familiarity with GMP and regulatory guidelines.
Production – Officer / Senior Officer
- Qualifications: B.Sc. or M.Sc. in Organic Chemistry
- Experience: 2–5 years
Skills:
- Experience in API or intermediate manufacturing.
- Knowledge of production processes, including reactor operations and scale-up.
- Familiarity with GMP and safety protocols in API production.
Candidate Preferences
We prioritize candidates with experience in API or intermediate manufacturing and familiarity with USFDA, EDQM, or EU-GMP standards. Proficiency in HPLC, GC, wet lab techniques, or synthesis lab operations is highly valued. Strong knowledge of GMP, QMS, and regulatory compliance will give applicants an edge.
Why Pursue a Career in Pharmaceutical Manufacturing?
The pharmaceutical industry offers stable, rewarding careers. At Oneiro Lifecare, you’ll work in a cutting-edge, GMP-certified facility, contributing to high-quality APIs and intermediates for global markets. Our roles provide growth opportunities in quality control, research & development, analytical development, and production.
Why Choose Gufic Biosciences?
Gufic Biosciences is renowned for its state-of-the-art sterile injectable facilities. Our Indore plant, among the largest globally for lyophilized injectables, fosters a dynamic and innovative work environment. We prioritize quality, innovation, and employee development, making us a premier choice for pharmaceutical professionals.
How to Prepare for the Interview
Arrive with all required documents and be ready to discuss your experience in API manufacturing, analytical techniques, or synthesis processes. Highlight expertise in HPLC, GC, wet lab analysis, or production operations.
Verified by Trusted HRs
The post is released by the Oneiro Lifecare LinkedIn page. Click here to visit the post
About Oneiro Lifecare Pvt. Ltd.
Established in 2004, Oneiro Lifecare is a quality-driven pharmaceutical company manufacturing APIs, pellets, and intermediates. Our Vadodara facility is ISO 9001:2008 and GMP-certified, with global accreditations from USFDA, EDQM, and EU-GMP. We are committed to innovation and excellence in drug development. Learn more at Oneiro Lifecare.
Join Our Team
Don’t miss this opportunity to advance your career with a leading pharmaceutical manufacturer. Attend our walk-in interview on July 12, 2025, at our Ekalbara facility in Vadodara. For those unable to attend, email your CV to recruitment@oneirolifecare.com.
Note: Oneiro Lifecare does not charge for job applications or interviews. Verify opportunities through our official website to avoid fraudulent communications.

