Unlock rewarding pharma jobs at Neuland Labs. QA jobs, QC jobs, production jobs, and engineering roles await skilled professionals. Elevate your pharmaceutical careers in India with global CDMO excellence.
Contents
About the Company
Neuland Laboratories Limited, founded in 1984, is a premier global API contract development and manufacturing organization (CDMO) headquartered in Hyderabad, India. With over 3,300 employees and a market cap exceeding $1.8 billion, the company excels in producing active pharmaceutical ingredients (APIs) and intermediates for biotech and pharma clients worldwide.
Committed to innovation, stringent regulatory compliance, and sustainable growth, Neuland delivers end-to-end solutions across Europe, the US, and beyond, earning trust through quality and cutting-edge technology.
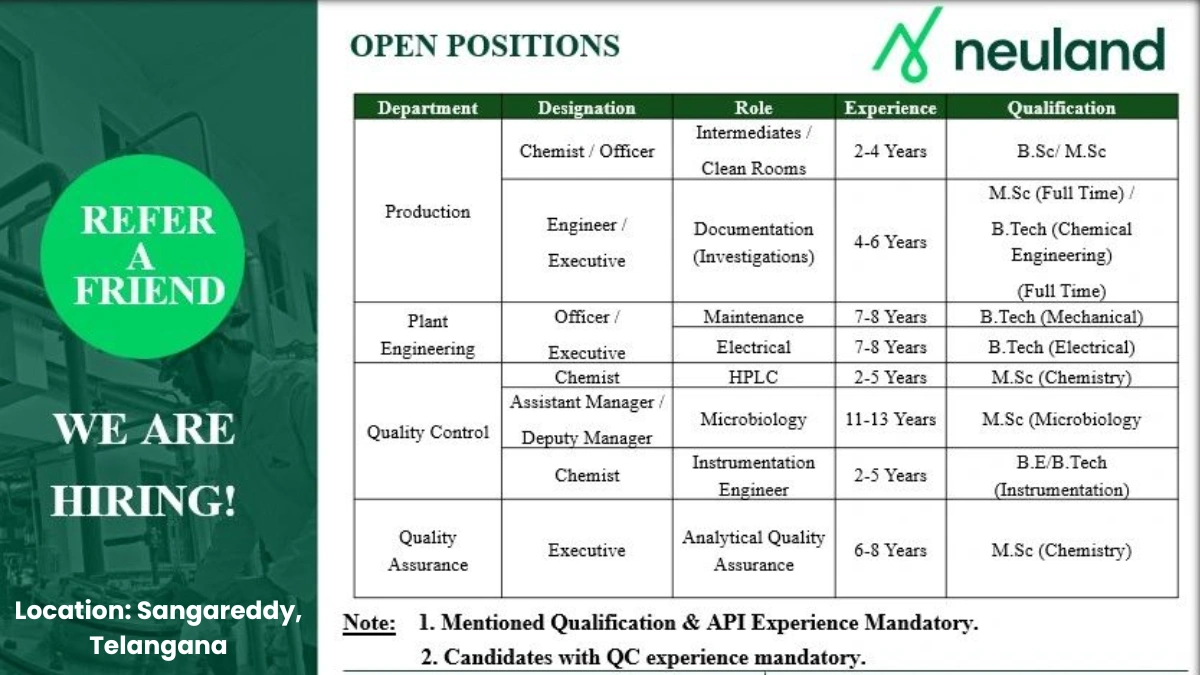
Job Details
- Company Name: Neuland Laboratories Limited
- Experience: Varies by role (2-13 years)
- Qualification: Varies (B.Sc./M.Sc., B.Tech/B.E. in relevant fields)
- Location: Unit-2, Plot No. 92-94, 257-259, IDA Pashamylaram Village, Patancheru Mandal, Sangareddy Dist, Telangana – 502319
- Work Type: On-site
Job Description
Neuland Laboratories is expanding its talented team across production, engineering, quality control, and assurance departments. These roles demand API experience and focus on precision in pharmaceutical manufacturing. Fresh opportunities arise for driven experts to drive innovation in API development.
Production Chemist/Officer
- Department: Production
- Market: Global API
- Role: Intermediates/Clean Rooms
- Experience: 2-4 Years
- Education/Qualification: B.Sc./M.Sc.
Production Engineer/Executive
- Department: Production
- Market: Global API
- Role: Documentation (Investigations)
- Experience: 4-6 Years
- Education/Qualification: M.Sc. (Full Time)/B.Tech (Chemical Engineering) (Full Time)
Plant Engineering Officer/Maintenance Executive
- Department: Plant Engineering
- Market: Global API
- Role: Electrical
- Experience: 7-8 Years
- Education/Qualification: B.Tech (Mechanical/Electrical)
Quality Control Chemist (HPLC)
- Department: Quality Control
- Market: Global API
- Role: HPLC Analysis
- Experience: 2-5 Years
- Education/Qualification: M.Sc. (Chemistry)
Quality Control Assistant Manager/Deputy Manager
- Department: Quality Control
- Market: Global API
- Role: Microbiology
- Experience: 11-13 Years
- Education/Qualification: M.Sc. (Microbiology)
Quality Control Chemist/Instrumentation Engineer
- Department: Quality Control
- Market: Global API
- Role: Instrumentation
- Experience: 2-5 Years
- Education/Qualification: B.E./B.Tech (Instrumentation)
Quality Assurance Executive
- Department: Quality Assurance
- Market: Global API
- Role: Analytical Quality Assurance
- Experience: 6-8 Years
- Education/Qualification: M.Sc. (Chemistry)
Skills/Qualifications
- Proficiency in API manufacturing and relevant technical fields
- Hands-on experience with GMP, HPLC, and microbiology protocols
- Strong analytical and problem-solving abilities
- API-specific knowledge mandatory; QC background essential for lab roles
- Excellent documentation and compliance skills
Key Responsibilities
- Conduct precise chemical analysis and testing
- Maintain clean room standards and documentation
- Investigate production discrepancies swiftly
- Perform electrical maintenance on plant equipment
- Oversee microbiological quality assessments
Benefits/Perks
- Accelerated career growth in a leading CDMO
- Continuous learning via advanced training programs
- Competitive salaries with performance incentives
- Inclusive work culture fostering collaboration
- Global exposure through international projects
How to Apply
Send your updated resume to ksoni@neulandlabs.com, specifying the position in the subject line. Highlight your API and QC experience.

For additional pharma jobs, explore Pharma Recruiter. Apply now—join Neuland’s innovative team and shape the future of pharmaceuticals!
Why You Should Join
Neuland Laboratories champions a culture of excellence, recognized for its employee-centric policies and industry awards in API innovation. Secure long-term stability in a thriving sector with robust growth trajectories. Embrace endless learning through mentorship and R&D immersion in a compliant, forward-thinking environment that pioneers affordable global healthcare solutions.
FAQs
What qualifications are required for these pharma jobs?
Relevant degrees like B.Sc./M.Sc. in Chemistry/Microbiology or B.Tech in Engineering; API experience is mandatory across roles.
How do I apply for positions at Neuland Labs?
Email your resume to ksoni@neulandlabs.com with the role name. QC experience is essential for quality positions.
What experience levels are needed for QA jobs and QC jobs?
Ranges from 2-13 years; production roles start at 2-4 years, while senior QC needs 11-13 years.
What growth opportunities exist in pharmaceutical careers in India at Neuland?
Rapid promotions, skill development, and global assignments ensure sustained advancement in API manufacturing.