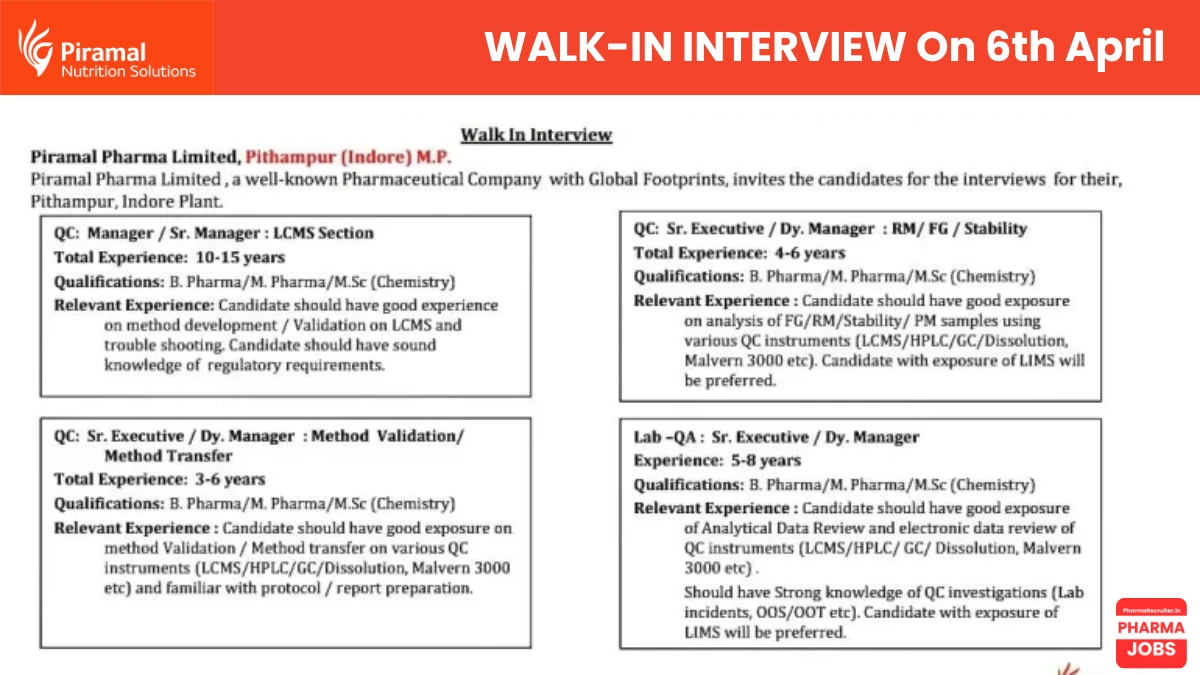
Piramal Pharma Limited, a renowned pharmaceutical company with a global footprint, is hosting walk-in interviews for multiple positions at our Pithampur (Indore) plant. With a commitment to innovation and excellence, we invite talented professionals to join our Quality Control (QC), Quality Assurance (QA), and Lab-QA teams. Don’t miss this opportunity to be part of our growth story!
Contents
Walk-In Interview Details
Date: Sunday, April 6, 2025
Time: 9:00 AM – 3:00 PM
Venue:
The Red Maple Mashal Hotel
Jhoomer Ghat, Rasalpura, Rau, Indore, Madhya Pradesh – 453446
Contact:
- Anuj Yagik: +91 6260600524
- Vijayraj Singh Chauhan: +91 6260322279
Open Positions
We’re hiring across Quality Control (QC), Quality Assurance (QA), and Lab-QA departments. Below are the details:
Quality Control (QC)
| Position | Experience | Qualifications | Key Skills & Experience |
|---|---|---|---|
| Sr. Executive / Dy. Manager – Microbiology | 4-8 years | M.Sc. (Microbiology/Biotech) | Microbial testing (MLT, Sterility, BET, PET), environmental/water monitoring, equipment handling (Autoclave, Vitek, TOC), LIMS preferred. |
| Manager / Sr. Manager – LCMS Section | 10-15 years | B.Pharma / M.Pharma / M.Sc. (Chemistry) | Method development/validation on LCMS, troubleshooting, regulatory knowledge. |
| Sr. Executive / Dy. Manager – Method Validation/Transfer | 3-6 years | B.Pharma / M.Pharma / M.Sc. (Chemistry) | Method validation/transfer on LCMS, HPLC, GC, Dissolution, Malvern 3000, protocol/report preparation. |
| Sr. Executive / Dy. Manager – RM/FG/Stability | 4-6 years | B.Pharma / M.Pharma / M.Sc. (Chemistry) | Analysis of RM/FG/Stability samples using LCMS, HPLC, GC, Dissolution, Malvern 3000, LIMS preferred. |

Quality Assurance (QA)
| Position | Experience | Qualifications | Key Skills & Experience |
|---|---|---|---|
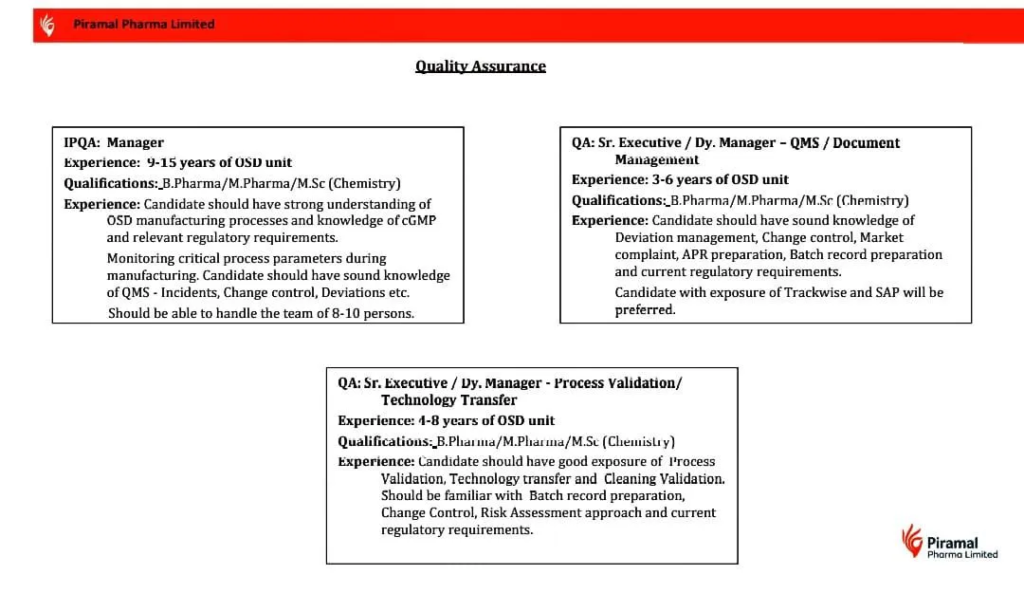
| Manager – IPQA | 9-15 years | B.Pharma / M.Pharma / M.Sc. (Chemistry) | OSD manufacturing, cGMP, QMS (incidents, change control, deviations), team management (8-10 people). |
| Sr. Executive / Dy. Manager – QMS/Document Management | 3-6 years | B.Pharma / M.Pharma / M.Sc. (Chemistry) | Deviation management, change control, market complaints, APR, batch records, Trackwise/SAP preferred. |
| Sr. Executive / Dy. Manager – Process Validation/Tech Transfer | 4-8 years | B.Pharma / M.Pharma / M.Sc. (Chemistry) | Process validation, tech transfer, cleaning validation, risk assessment, batch records, regulatory knowledge. |
Lab-QA
| Position | Experience | Qualifications | Key Skills & Experience |
|---|---|---|---|
| Sr. Executive / Dy. Manager | 5-8 years | B.Pharma / M.Pharma / M.Sc. (Chemistry) | Analytical/electronic data review (LCMS, HPLC, GC, Dissolution, Malvern 3000), QC investigations (OOS/OOT), LIMS preferred. |
Why Join Piramal Pharma?
Piramal Pharma Limited offers:
- A global platform to showcase your expertise.
- Exposure to advanced technologies and regulatory standards.
- A collaborative environment focused on quality and innovation.
Who Should Attend?
We’re looking for candidates with:
- Relevant experience in pharmaceutical QC, QA, or Lab-QA (OSD or microbiology focus).
- Strong technical skills and familiarity with regulatory requirements.
- Willingness to contribute to a dynamic team.
How to Participate
Walk-In Process
- When: April 6, 2025, 9:00 AM – 3:00 PM.
- Where: The Red Maple Mashal Hotel, Indore.
- Bring: Your updated CV and relevant documents.
Contact Information
- Phone: +91 6260600524 (Anuj Yagik) / +91 6260322279 (Vijayraj Singh Chauhan)
- Location: Piramal Pharma Limited, Pithampur, Indore, Madhya Pradesh
Be Part of Our Legacy
Join Piramal Pharma Limited and contribute to our mission of delivering world-class pharmaceutical solutions. Walk in on April 6th and take the next step in your career. We look forward to meeting you!