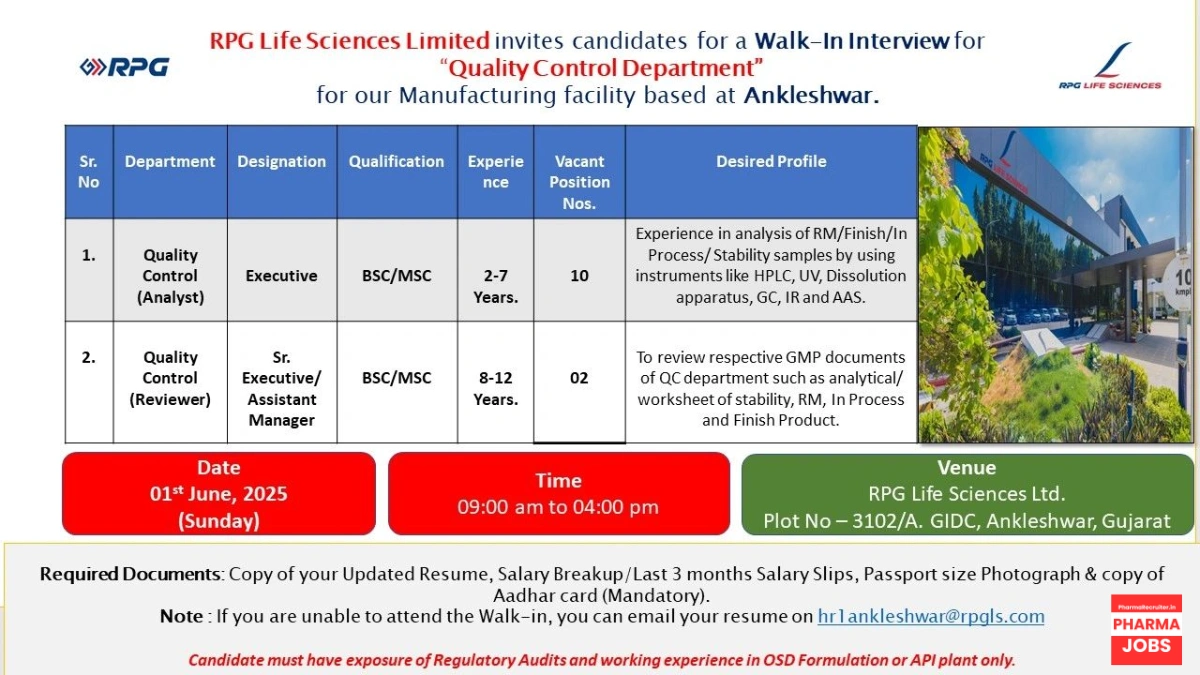
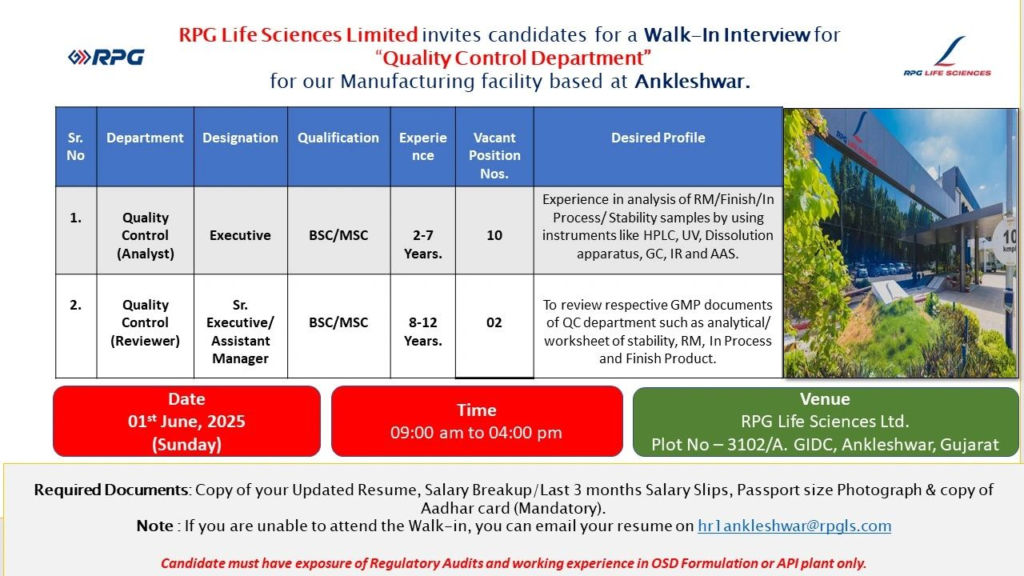
RPG Life Sciences Limited, a leading pharmaceutical company, is conducting a walk-in interview for talented professionals to join our Quality Control Department at our manufacturing facility in Ankleshwar, Gujarat. This is a prime opportunity for candidates with expertise in pharmaceutical quality control to contribute to a dynamic team in a reputed organization.
Contents
Event Details
- Date: Sunday, June 1, 2025
- Time: 09:00 AM to 04:00 PM
- Venue:
RPG Life Sciences Ltd
Plot No. 3102/A, GIDC, Ankleshwar, Gujarat
View Location on Google Maps
Open Positions and Requirements
We are seeking skilled professionals for the Quality Control Department with experience in Oral Solid Dosage (OSD) formulation or API plant operations. Below is a detailed breakdown of the roles, qualifications, experience, and desired skills:
| Sr. No | Department | Designation | Qualification | Experience | Vacant Positions | Desired Profile |
|---|---|---|---|---|---|---|
| 1 | Quality Control (Analyst) | Executive | B.Sc. / M.Sc. | 2–7 Years | 10 | Experience in analysis of Raw Material (RM), Finished Products, In-Process, and Stability samples using instruments like HPLC, UV, Dissolution Apparatus, GC, IR, and AAS. |
| 2 | Quality Control (Reviewer) | Sr. Executive / Assistant Manager | B.Sc. / M.Sc. | 8–12 Years | 2 | Expertise in reviewing GMP documents such as analytical worksheets for stability, RM, In-Process, and Finished Products. |
Key Requirements
- Candidates must have exposure to regulatory audits (e.g., USFDA, MHRA).
- Experience in OSD formulation or API plant is mandatory.
- Familiarity with Good Manufacturing Practices (GMP) and quality control processes.
Key Responsibilities
- Analyst (Executive):
- Conduct analysis of RM, Finished Products, In-Process, and Stability samples.
- Operate and maintain analytical instruments like HPLC, UV, Dissolution Apparatus, GC, IR, and AAS.
- Ensure compliance with regulatory standards and GMP guidelines.
- Reviewer (Sr. Executive/Assistant Manager):
- Review GMP documents including analytical worksheets for stability, RM, In-Process, and Finished Products.
- Ensure accuracy and compliance with regulatory audit requirements.
- Support quality control processes in OSD formulation or API plant settings.
Mandatory Documents to Bring
To facilitate a smooth interview process, please bring the following documents:
- Updated resume
- Salary breakup or last 3 months’ salary slips
- Passport-size photograph
- Copy of Aadhar card (mandatory)
How to Apply
- In-Person: Attend the walk-in interview at the specified venue with all required documents.
- Outstation Candidates: Email your resume to hr1ankleshwar@rpgls.com.
Why Join RPG Life Sciences?
- Industry Leader: Be part of a trusted name in the pharmaceutical industry with a focus on OSD formulations and APIs.
- Career Growth: Opportunities to work on regulatory-compliant projects and enhance your expertise in quality control.
- Collaborative Environment: Join a team dedicated to innovation, quality, and excellence in pharmaceutical manufacturing.
About RPG Life Sciences Ltd
Established as a pioneer in the pharmaceutical sector, RPG Life Sciences specializes in API manufacturing and formulation development, delivering high-quality products globally. With a commitment to innovation and regulatory compliance, we strive to make a positive impact on healthcare.