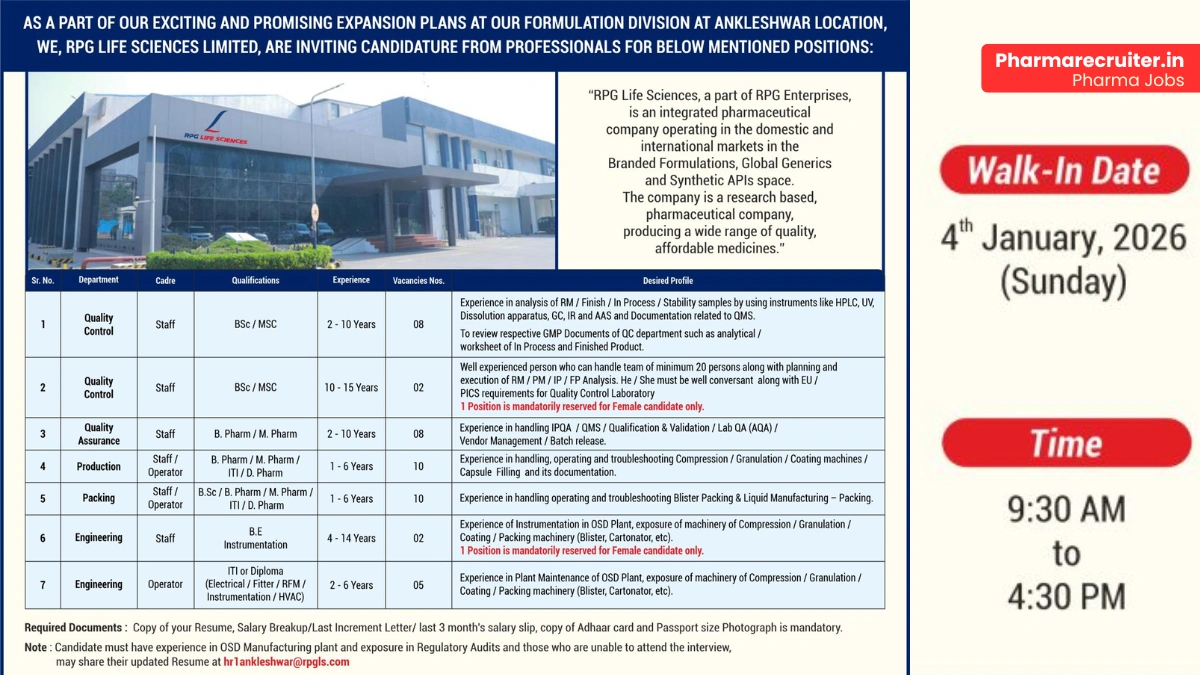
Join RPG Life Sciences for rewarding pharmaceutical careers in India! Exciting walk-in interview on January 4, 2026, for QC jobs, QA jobs, production jobs, and engineering roles in Ankleshwar formulation plant.
Contents
About the Company
RPG Life Sciences Limited, a proud part of the esteemed RPG Enterprises, is an integrated pharmaceutical company with a strong presence in domestic and international markets. The company excels in Branded Formulations, Global Generics, and Synthetic APIs segments.
Known for its research-driven approach, RPG Life Sciences consistently delivers high-quality, affordable medicines while maintaining strict regulatory compliance. With a focus on innovation, global standards, and sustainable growth, it offers professionals a dynamic platform to build long-term pharmaceutical careers in India.
Job Details
- Company Name: RPG Life Sciences Limited
- Experience: 1–15 years (varies by role)
- Qualification: B.Sc/M.Sc (Chemistry), B.Pharm/M.Pharm, ITI, D.Pharm, B.E (Instrumentation), Diploma (Electrical/Fitter/RFM/Instrumentation/HVAC)
- Location: Ankleshwar, Gujarat
- Work Type: On-site
Job Description
RPG Life Sciences is expanding its Formulation Division at Ankleshwar and invites experienced professionals for multiple roles across Quality Control, Quality Assurance, Production, Packing, and Engineering departments. These positions demand hands-on expertise in OSD (Oral Solid Dosage) manufacturing and exposure to regulatory audits.
Quality Control
- Department: Quality Control
- Role: Staff (Analyst/Reviewer/Section Head level)
- Experience: 2–10 years (8 vacancies); 10–15 years (2 vacancies)
- Education/Qualification: B.Sc/M.Sc (Chemistry)
Quality Assurance
- Department: Quality Assurance
- Role: Staff
- Experience: 2–10 years
- Education/Qualification: B.Pharm/M.Pharm
- Vacancies: 8 (1 position mandatorily reserved for female candidate)
Production
- Department: Production
- Role: Staff/Operator
- Experience: 1–6 years
- Education/Qualification: B.Pharm/M.Pharm/ITI/D.Pharm
- Vacancies: 10
Packing
- Department: Packing
- Role: Staff/Operator
- Experience: 1–6 years
- Education/Qualification: B.Sc/B.Pharm/M.Pharm/ITI/D.Pharm
- Vacancies: 10
Engineering (Instrumentation)
- Department: Engineering
- Role: Staff
- Experience: 4–14 years
- Education/Qualification: B.E (Instrumentation)
- Vacancies: 2 (1 position mandatorily reserved for female candidate)
Engineering (Maintenance)
- Department: Engineering
- Role: Operator
- Experience: 2–6 years
- Education/Qualification: ITI or Diploma (Electrical/Fitter/RFM/Instrumentation/HVAC)
- Vacancies: 5
Skills/Qualifications
- Hands-on experience in analysis using HPLC, UV, Dissolution, GC, IR, AAS
- Proficiency in GMP documentation and QMS
- Knowledge of EU/PICS requirements for QC laboratory (for senior roles)
- Experience in IPQA, Validation, Qualification, Vendor Management, Batch Release
- Operation and troubleshooting of Compression, Granulation, Coating, Capsule Filling machines
- Expertise in Blister Packing and Liquid Manufacturing-Packing lines
- Instrumentation and maintenance exposure in OSD plant machinery
- Mandatory experience in OSD manufacturing and regulatory audits
Key Responsibilities
- Analyse RM/PM/In-Process/Finished/Stability samples
- Review GMP documents and analytical worksheets
- Lead team of 20+ members with planning and execution
- Handle IPQA, QMS, validation, and batch release activities
- Operate and troubleshoot granulation, compression, coating machines
- Manage blister packing and secondary packing operations
- Maintain instrumentation and plant machinery
- Ensure compliance during regulatory audits
Benefits/Perks
- Competitive salary and performance incentives
- Structured career growth path
- Continuous learning and training opportunities
- Exposure to global regulatory standards
- Positive and inclusive work culture
- Long-term stability with a reputed RPG Group company
How to Apply
Candidates with relevant OSD plant experience are encouraged to apply. Carry updated resume, salary breakup/last increment letter, last 3 months’ salary slips, Aadhaar card copy, and passport-size photograph.
Verified Post
Verification: To confirm the legitimacy of this posting, you can view the original announcement on the RPG Life Science LinkedIn page.

Unable to attend? Email your updated resume to hr.ankleshwar@rpgls.com. For more pharma job opportunities across India, visit Pharma Recruiter. Take the next step in your pharmaceutical career — apply today!
Walk-in Interview Details
- Date: January 4, 2026 (Sunday)
- Time: 9:30 AM to 4:30 PM
- Venue: Hotel Woodlands, National Highway 8, Near Tejpal Motors, Balitha, Vapi, Gujarat – 396191
- Note: RPG Group never charges any fees during recruitment. Report any such requests to bhavin.desai@rpgls.com
Why You Should Join
RPG Life Sciences offers more than just a job — it provides a platform for meaningful contribution in a research-based organisation committed to quality and innovation. With a strong emphasis on compliance, employee development, and global exposure, professionals here enjoy stable, rewarding careers in a supportive environment that values excellence and work-life balance.
FAQs
What is the eligibility criteria for these positions?
Candidates must have relevant experience in OSD formulation manufacturing and exposure to regulatory audits (USFDA/MHRA/EU etc.).
Is the walk-in interview mandatory?
No. Candidates unable to attend can email their resume to hr.ankleshwar@rpgls.com with relevant documents.
Are there reservations for specific candidates?
Yes, one position each in Quality Assurance and Engineering (Instrumentation) is mandatorily reserved for female candidates.
What growth opportunities are available?
RPG Life Sciences offers structured career progression, continuous training, and exposure to international markets and advanced technologies.