Senores Pharmaceuticals Limited, a trailblazer in Active Pharmaceutical Ingredient (API) manufacturing, is hosting a walk-in interview on July 27, 2025, for dynamic professionals. Our state-of-the-art facility in Mehsana, Gujarat, is designed to meet global standards, including USFDA and EU regulations.
Join us to contribute to innovative healthcare solutions and advance your career. Visit www.senorespharma.com for more details.
Contents
Why Senores Pharmaceuticals?
Senores is committed to producing high-quality APIs for regulated markets, with a presence in over 40 countries. Our 230,000 sq. ft. greenfield facility in Mehsana, boasting a 100 MT annual capacity, strengthens our global competitiveness in CDMO/CMO services. Be part of a research-driven team shaping the future of pharmaceuticals.
Open Positions and Requirements
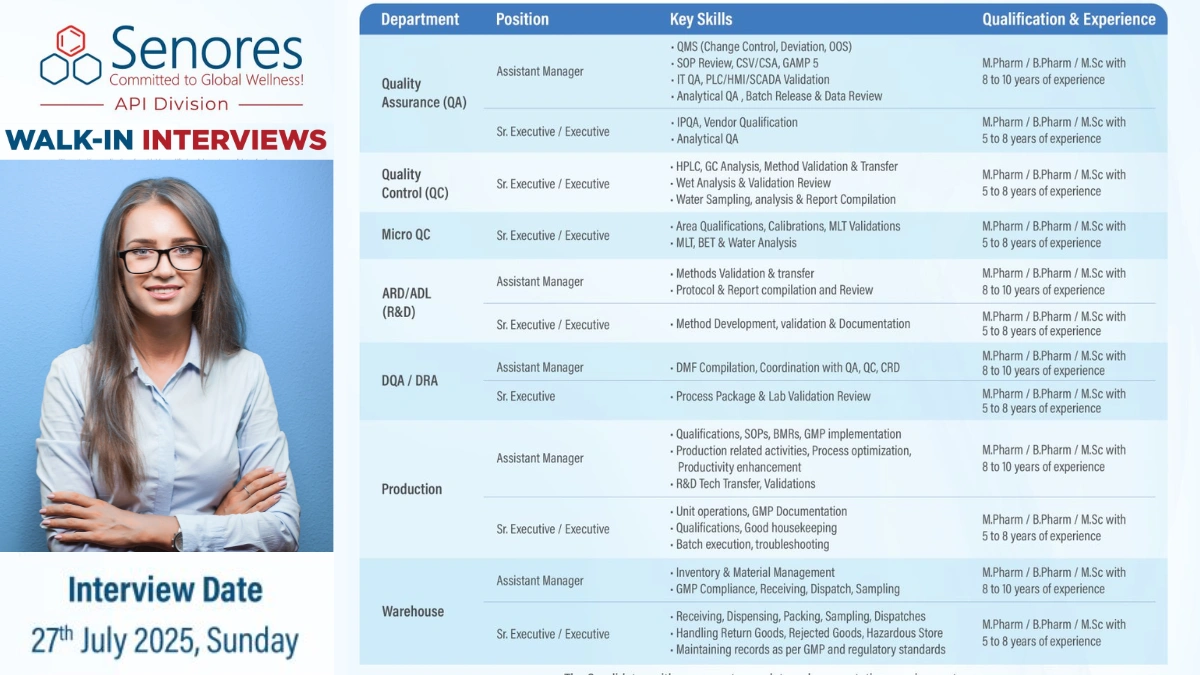
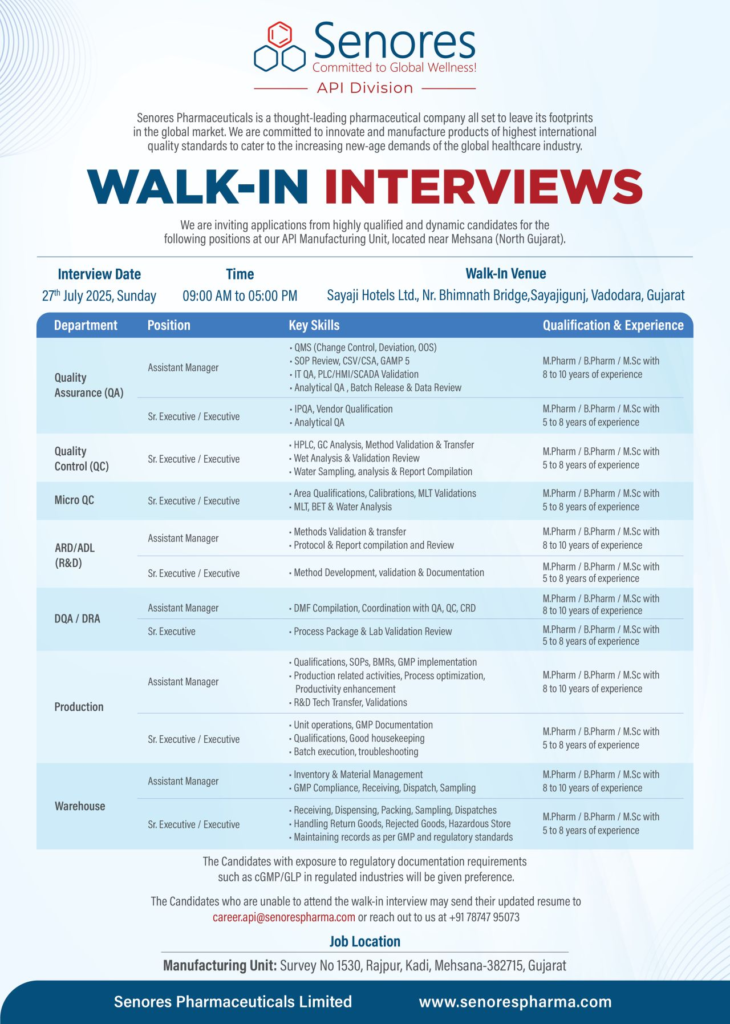
Quality Assurance (QA) – Assistant Manager, Sr. Executive/Executive
- Qualification: M.Pharm/B.Pharm/M.Sc
- Experience: 5-10 years
- Skills: QMS (Change Control, Deviation, OOS), SOP Review, CSV/CSA, GAMP 5, IT QA, PLC/HMI/SCADA Validation, Analytical QA, Batch Release, IPQA, Vendor Qualification.
Quality Control (QC) – Sr. Executive/Executive
- Qualification: M.Pharm/B.Pharm/M.Sc
- Experience: 5-8 years
- Skills: HPLC, GC Analysis, Method Validation & Transfer, Wet Analysis, Validation Review, Water Sampling, Analysis & Report Compilation.
Micro QC – Sr. Executive/Executive
- Qualification: M.Pharm/B.Pharm/M.Sc
- Experience: 5-8 years
- Skills: Area Qualifications, Calibrations, MLT Validations, BET & Water Analysis.
AR&D/ADL (R&D) – Assistant Manager, Sr. Executive/Executive
- Qualification: M.Pharm/B.Pharm/M.Sc
- Experience: 5-10 years
- Skills: Method Development, Validation, Documentation, DMF Compilation, QA/QC/CRD Coordination.
DQA/DRA – Assistant Manager, Sr. Executive
- Qualification: M.Pharm/B.Pharm/M.Sc
- Experience: 5-10 years
- Skills: Process Package & Lab Validation Review, DMF Compilation.
Production – Assistant Manager, Sr. Executive/Executive
- Qualification: M.Pharm/B.Pharm/M.Sc
- Experience: 5-10 years
- Skills: Qualifications, SOPs, BMRs, GMP Implementation, Process Optimization, R&D Tech Transfer, Unit Operations, Batch Execution, Troubleshooting.
Warehouse – Assistant Manager, Sr. Executive/Executive
- Qualification: M.Pharm/B.Pharm/M.Sc
- Experience: 5-10 years
- Skills: Inventory & Material Management, GMP Compliance, Receiving, Dispensing, Packing, Sampling, Handling Return/Rejected Goods, Hazardous Store Management.
Walk-In Interview Details
- Date: Sunday, July 27, 2025
- Time: 9:00 AM to 5:00 PM
- Venue: Sayaji Hotels Ltd., Near Bhimnath Bridge, Sayajigunj, Vadodara, Gujarat
- Work Location: Survey No. 1530, Rajpur, Kadi, Mehsana-382715, Gujarat
- Contact Email: career.api@senorespharma.com
- Contact Number: +91 78747 95073
Documents to Bring
Please bring the following:
- Updated CV highlighting cGMP/GLP experience.
- Photocopies of educational certificates (M.Pharm/B.Pharm/M.Sc).
- PAN and Aadhaar card copies.
- Last 3 months’ payslips or 6 months’ bank statements (if applicable).
Candidate Requirements
We prioritize candidates with exposure to regulatory documentation (cGMP/GLP) and experience in regulated industries (USFDA, EU). Proficiency in HPLC, GC, method validation, or GMP compliance is essential. Candidates should be dynamic, with 5-10 years of experience in API manufacturing or related fields.
Why Join Senores?
Senores offers a platform to work on complex generics and APIs, with opportunities to engage in global markets. Our Mehsana facility, inaugurated in February 2025, enhances our CDMO/CMO capabilities, providing exposure to cutting-edge technologies and a 4.4/5 work-life balance rating.
How to Apply
Attend the walk-in interview in Vadodara with all required documents. If unable to attend, email your resume to career.api@senorespharma.com or contact +91 78747 95073. Highlight your experience in regulatory compliance and API processes.
About Senores Pharmaceuticals
Headquartered in Ahmedabad, Senores is a global leader in developing and manufacturing complex generics and APIs. With facilities in Atlanta (USFDA-approved) and India, we serve over 40 countries, focusing on quality and affordability. Our recent acquisition of 14 ANDAs underscores our growth trajectory.
Seize This Opportunity!
Join Senores Pharmaceuticals on July 27, 2025, to advance your career in API manufacturing. Whether in QA, QC, Production, or Warehouse, our Mehsana facility offers a dynamic environment to excel. Apply now and contribute to global wellness with Senores!