SMS Pharmaceuticals Ltd., a leading API manufacturer in India with cGMP and WHO-GMP compliant facilities, is hosting a walk-in interview for multiple roles at its Unit-II facility in Bachupally, Hyderabad. Established in 1990, SMS Pharma is a global player with a strong R&D and manufacturing team, exporting to 70+ countries and rated 3.9/5 for job security (AmbitionBox, 200+ reviews). The company specializes in APIs for oncology, anti-viral, and cardiovascular therapies, with USFDA and EDQM approvals.
Contents
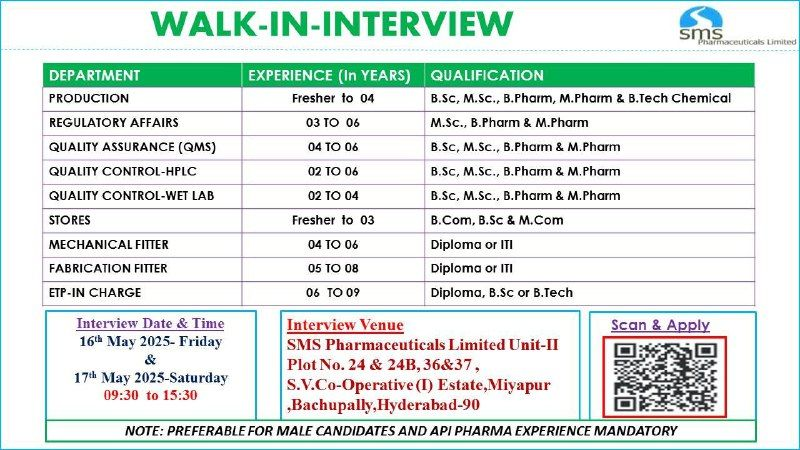
Event Details
- Interview Dates: 16th May 2025 (Friday) & 17th May 2025 (Saturday)
- Time: 9:30 AM to 3:30 PM
- Venue: SMS Pharmaceuticals Ltd., Unit-II, Plot No. 24, 24B, 36 & 37, S.V. Co-Operative Industrial Estate, Bachupally, Miyapur, Hyderabad – 500090, Telangana, India
- Application Process: Attend the walk-in with required documents or scan the QR code (from official communication) to apply. Email CV to ehs@smspharma.com for pre-registration.
- Note: Preferable for male candidates with API pharma experience (mandatory).
- Contact: +91-40-25259999 for queries
Job Opportunities
1. Production
- Experience: Fresher to 4 years
- Qualification: B.Sc., M.Sc., B.Pharm, M.Pharm, B.Tech (Chemical)
- Salary: ₹2.5–5.0 Lakhs/year (estimated, Hyderabad, industry standards)
Responsibilities:
- Operate API production equipment (reactors, centrifuges, dryers) in cGMP environment.
- Monitor processes, ensure batch compliance, and maintain BMR/BPR records.
- Support scale-up and process optimization for API manufacturing.
- Adhere to USFDA and WHO-GMP standards.
Preferences:
- Knowledge of SSR/GLLegacy API manufacturing processes.
- Exposure to QMS (deviations, CAPA) and data integrity.
2. Regulatory Affairs
- Experience: 3–6 years
- Qualification: M.Sc., B.Pharm, M.Pharm
- Salary: ₹4.5–8.0 Lakhs/year (estimated)
Responsibilities:
- Prepare and submit DMFs, CTDs, CEPs, and LOAs to regulatory bodies (USFDA, EDQM).
- Ensure compliance with ICH Q7 and 21 CFR Part 11.
- Coordinate with cross-functional teams for regulatory audits.
- Maintain regulatory databases and documentation.
Preferences:
- Experience in API regulatory submissions for US/EU markets.
- Familiarity with eCTD and query response management.
3. Quality Assurance (QMS)
- Experience: 4–6 years
- Qualification: B.Sc., M.Sc., B.Pharm, M.Pharm
- Salary: ₹4.5–8.0 Lakhs/year (estimated)
Responsibilities:
- Manage QMS elements (OOS, CAPA, deviations, change control) for API production.
- Conduct IPQA, validation, and qualification per cGMP.
- Review BMR/BPR and ensure USFDA audit readiness.
- Maintain GDP and data integrity standards.
Preferences:
- Experience in API plants with USFDA/EDQM approvals.
- Proficiency in QMS software (e.g., Trackwise).
4. Quality Control – HPLC
- Experience: 2–6 years
- Qualification: B.Sc., M.Sc., B.Pharm, M.Pharm
- Salary: ₹3.5–6.5 Lakhs/year (estimated)
Responsibilities:
- Perform HPLC-based analysis for API testing (assay, impurities).
- Validate analytical methods per ICH Q2.
- Operate and maintain HPLC systems, ensuring 21 CFR Part 11 compliance.
- Document results and support stability studies.
Preferences:
- Hands-on experience with Agilent/Waters HPLC systems.
- Knowledge of method development and troubleshooting.
5. Quality Control – Wet Lab
- Experience: 2–4 years
- Qualification: B.Sc., M.Sc., B.Pharm, M.Pharm
- Salary: ₹3.0–5.5 Lakhs/year (estimated)
Responsibilities:
- Conduct wet chemistry analysis (titration, LOD, sulfated ash).
- Perform raw material, in-process, and finished product testing.
- Maintain lab records per GLP and GDP.
- Support QC documentation for USFDA audits.
Preferences:
- Experience in API wet lab testing.
- Familiarity with pharmacopoeial standards (USP, EP).
6. Stores
- Experience: Fresher to 3 years
- Qualification: B.Com, B.Sc., M.Com
- Salary: ₹2.0–4.0 Lakhs/year (estimated)
Responsibilities:
- Manage receipt, storage, and dispensing of RM, PM, and FG.
- Maintain inventory records in SAP, ensuring FIFO/FEFO.
- Prepare GRN, RGP/NRGP, and coordinate with QC/production.
- Ensure GDP compliance in warehousing.
Preferences:
- Experience in API warehouse operations.
- Basic SAP/ERP proficiency.
7. Mechanical Fitter
- Experience: 4–6 years
- Qualification: Diploma or ITI
- Salary: ₹2.5–4.5 Lakhs/year (estimated)
Responsibilities:
- Perform preventive maintenance and repairs on API equipment (pumps, reactors).
- Troubleshoot mechanical issues in centrifuges, dryers, and gear boxes.
- Maintain maintenance logs per cGMP standards.
- Support USFDA audit readiness.
Preferences:
- Experience in API plant maintenance.
- Knowledge of predictive maintenance techniques.
8. Fabrication Fitter
- Experience: 5–8 years
- Qualification: Diploma or ITI
- Salary: ₹3.0–5.0 Lakhs/year (estimated)
Responsibilities:
- Fabricate and assemble API plant piping and structural components.
- Perform welding, cutting, and fitting per engineering drawings.
- Ensure compliance with cGMP and safety standards.
- Support equipment installation and modification.
Preferences:
- Experience in API facility fabrication.
- Proficiency in TIG/MIG welding for SS/MS.
9. ETP-In Charge
- Experience: 6–9 years
- Qualification: Diploma, B.Sc., B.Tech
- Salary: ₹4.0–7.0 Lakhs/year (estimated)
Responsibilities:
- Manage Effluent Treatment Plant (ETP) operations for API facility.
- Ensure compliance with CPCB/SPCB regulations.
- Monitor treatment processes (primary, secondary, tertiary).
- Maintain ETP documentation and support audits.
Preferences:
- Experience in API plant ETP operations.
- Knowledge of ZLD (Zero Liquid Discharge) systems.
Why Join SMS Pharmaceuticals?
- Work in a USFDA/EDQM-approved API manufacturing facility in Hyderabad’s Genome Valley.
- Contribute to a global portfolio of 100+ APIs for critical therapies.
- Rated 4.0/5 for skill development, with training in cGMP and regulatory compliance (AmbitionBox).
- Join a 1,000+ strong team with a ₹1,200 Cr+ turnover and 30+ years of legacy.
- Career growth in a company exporting to 70+ countries with 6 manufacturing units.
How to Prepare and Attend
Documents to Carry:
- Updated CV (highlighting API experience)
- Academic mark sheets (B.Sc., M.Sc., B.Pharm, M.Pharm, B.Tech, Diploma, ITI, etc.)
- Experience certificates
- Last 3 months’ payslips and bank statement
- Aadhaar, PAN copies
- 2 passport-size photographs
Preparation Tips:
- Production: Study API manufacturing processes (SSR, GLR, centrifugation). Be ready for questions on BMR/BPR and cGMP.
- Regulatory Affairs: Review DMF/CTD preparation and ICH Q7. Prepare for USFDA query handling scenarios.
- QA-QMS: Focus on QMS tools (Trackwise), ICH Q7, and audit preparedness.
- QC-HPLC/Wet Lab: Brush up on HPLC method validation and wet chemistry techniques. Know USP/EP standards.
- Stores: Demonstrate SAP proficiency and GDP knowledge. Discuss FIFO/FEFO practices.
- Mechanical/Fabrication Fitter: Highlight equipment maintenance or welding experience in API plants. Study cGMP compliance.
- ETP-In Charge: Review CPCB/SPCB norms and ETP process flows. Discuss ZLD experience.
- Interview Process: Expect technical questions, practical assessments (e.g., HPLC operation, QMS scenarios), and HR discussion. Telugu proficiency is a plus.
Important Notes
- Eligibility: Candidates with API pharma experience are mandatory. Male candidates preferred due to shift-based roles.
- Fraud Alert: SMS Pharma does not charge fees for recruitment. Verify communications via ehs@smspharma.com or hr@smspharma.com. Report suspicious activity to +91-40-25259999.
- Previous Applicants: Candidates interviewed in the last 6 months at SMS Pharma should not reapply.
About SMS Pharmaceuticals
Founded in 1990, SMS Pharmaceuticals Ltd. is a Hyderabad-based global leader in API manufacturing, with 6 units across Telangana and Andhra Pradesh. With a ₹1,200 Cr+ turnover, 1,000+ employees, and approvals from USFDA, EDQM, and WHO-GMP, SMS serves 70+ countries. Its R&D center, recognized by DSIR, drives innovation in oncology, anti-viral, and cardiovascular APIs, making it a top choice for pharma professionals.
Join Our Mission
Join SMS Pharmaceuticals’ mission to deliver high-quality APIs that improve global healthcare! Attend the walk-in interview on 16th–17th May 2025 in Hyderabad and grow with a world-class API leader!


