Discover top pharma jobs in India with SP Accure Labs’ walk-in interview on September 27, 2025! Explore injectables, OSD production, QA, and QC opportunities in Telangana.
Contents
About the Company
SP Accure Labs Pvt. Ltd. (SPAL), founded in 2015, is a fully integrated, quality-led pharmaceutical company headquartered in Hyderabad, India. Specializing in oncology formulations, SPAL excels in research, manufacturing, and marketing high-quality specialty products for global markets.
With state-of-the-art facilities designed for USFDA, MHRA, and PIC/S approvals, the company commits to innovation, accessibility, and patient-centric solutions. Employing advanced technologies and a collaborative approach, SP Accure Labs fosters pharmaceutical careers that drive breakthroughs in anticancer therapies and beyond.
Job Details
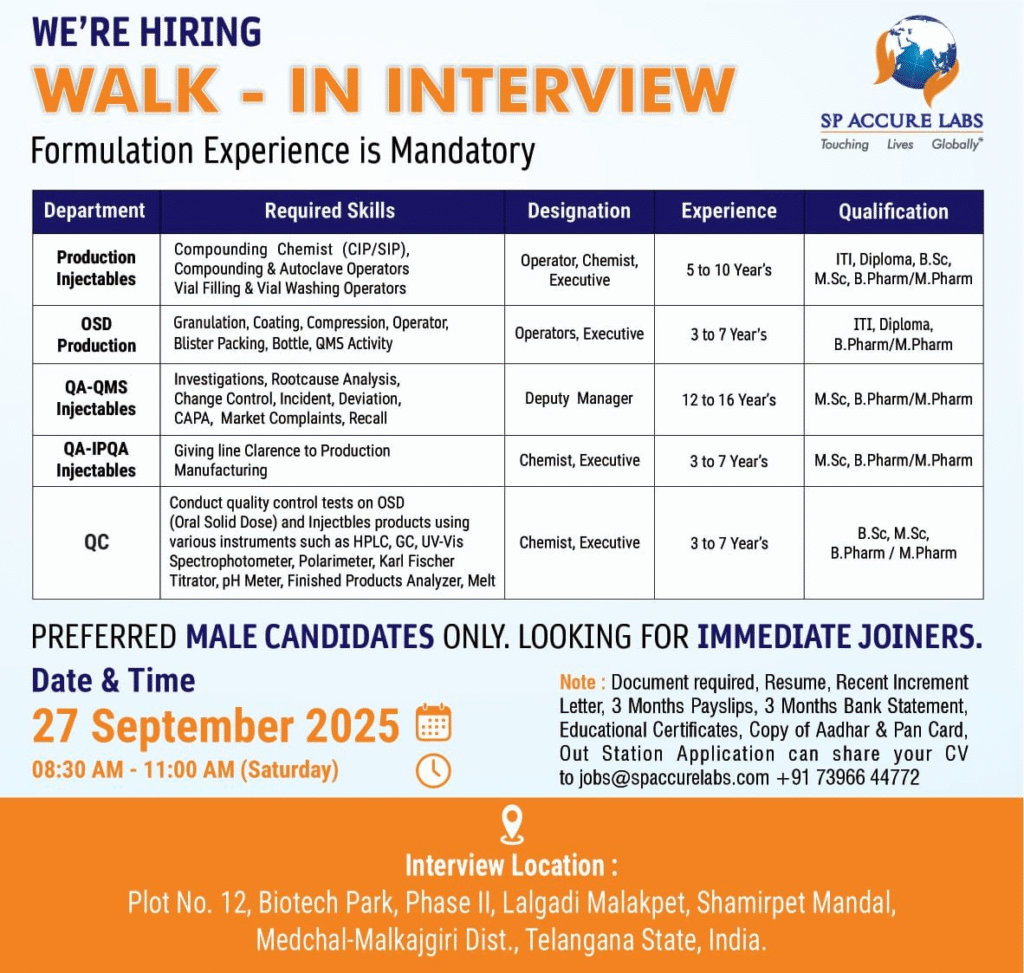
- Company Name: SP Accure Labs Pvt. Ltd.
- Experience: 3–16 years
- Qualification: ITI, Diploma, B.Sc., M.Sc., B.Pharm, M.Pharm
- Location: Plot No. 12, Biotech Park, Phase II, Lalgadi Malakpet, Shamirpet Mandal, Medchal-Malkajgiri Dist., Telangana, India
- Work Type: On-site, Full-time (formulation experience mandatory; immediate joiners preferred; male candidates only)
Job Description
SP Accure Labs is conducting a walk-in interview to hire for key roles in production, quality assurance, and quality control at its oncology-focused facility in Hyderabad. These pharma jobs require hands-on formulation experience in injectables and OSD, supporting global regulatory compliance and innovative drug manufacturing.
Production Injectables
- Department: Production Injectables
- Role: Compounding Chemist (CIP/SIP), Compounding & Autoclave Operators, Vial Filling & Vial Washing Operators
- Experience: 5–10 years
- Education/Qualification: ITI, Diploma, B.Sc., M.Sc., B.Pharm/M.Pharm
OSD Production
- Department: OSD Production
- Role: Granulation, Coating, Compression, Operators, Blister Packing, Bottle, QMS Activity
- Experience: 3–7 years
- Education/Qualification: ITI, Diploma, B.Pharm/M.Pharm
QA-QMS Injectables
- Department: QA-QMS Injectables
- Role: Investigations, Rootcause Analysis, Change Control, Incident, Deviation, CAPA, Market Complaints, Recall
- Experience: 12–16 years
- Education/Qualification: M.Sc., B.Pharm/M.Pharm
QA-IPQA Injectables
- Department: QA-IPQA Injectables
- Role: Giving line Clearance to Production Manufacturing
- Experience: 3–7 years
- Education/Qualification: M.Sc., B.Pharm/M.Pharm
QC
- Department: Quality Control
- Role: Chemist, Executive (Conduct quality control tests on OSD and Injectables using HPLC, GC, UV-Vis, Polarimeter, Karl Fischer, pH Meter, Finished Products Analyzer, Melt)
- Experience: 3–7 years
- Education/Qualification: B.Sc., M.Sc., B.Pharm/M.Pharm
Skills/Qualifications
- Proven formulation experience in injectables (CIP/SIP, compounding, autoclave, vial operations) and OSD (granulation, compression, coating, packing)
- Expertise in QA-QMS: root cause analysis, CAPA, deviations, change controls, and market complaints handling
- Proficiency in IPQA: line clearance, production oversight, and compliance checks
- Hands-on with QC instruments: HPLC, GC, UV-Vis Spectrophotometer, Polarimeter, Karl Fischer Titrator, pH Meter
- Relevant qualifications: ITI/Diploma for operators; B.Sc./M.Sc./B.Pharm/M.Pharm for chemists/executives
- 3–16 years in pharma manufacturing with GMP and regulatory exposure
- Strong analytical, documentation, and problem-solving skills in fast-paced settings
Key Responsibilities
- Perform compounding, autoclaving, vial filling/washing, and sterile operations in injectables production
- Execute granulation, compression, coating, blister/bottle packing, and QMS activities in OSD
- Lead investigations, root cause analysis, CAPA, and handle deviations/incidents in QA-QMS
- Conduct line clearance, in-process checks, and ensure manufacturing compliance in IPQA
- Run QC tests on OSD/injectables using HPLC, GC, UV-Vis, and other analytical tools
- Maintain accurate records, adhere to safety protocols, and support regulatory audits
- Collaborate across teams to meet production targets and quality standards
Benefits/Perks
- Competitive salaries for immediate joiners in India’s oncology pharma sector
- Career advancement in a USFDA/MHRA-compliant facility focused on innovation
- Training in advanced formulation and analytical techniques
- Supportive work culture emphasizing quality and global impact
- Health insurance, on-site amenities, and professional development programs
- Opportunities to contribute to life-saving oncology products worldwide
How to Apply
For outstation candidates, email your CV to jobs@spaccurelabs.com with the subject “Application for [Role/Department] – Hyderabad“.
Verified Post
The post is released by the SP Accure Labs LinkedIn page. Click here to visit the post
For more pharma job resources, visit Pharma Recruiter. Contact +91 73966 44772 for queries—apply now!
Walk-in Interview Details
- Date: September 27, 2025 (Saturday)
- Time: 08:30 AM to 11:00 AM
- Venue: Plot No. 12, Biotech Park, Phase II, Lalgadi Malakpet, Shamirpet Mandal, Medchal-Malkajgiri Dist., Telangana State, India
- Contact/Email: jobs@spaccurelabs.com, +91 73966 44772
Why You Should Join
SP Accure Labs provides a premier platform for pharmaceutical careers in oncology, where your formulation expertise drives global access to high-quality medicines. In a cutting-edge, regulatory-approved facility, collaborate on innovative therapies while advancing from operator to deputy manager roles.
With a commitment to quality and patient impact, enjoy stability, skill enhancement, and a male-focused hiring approach for immediate contributions. Join SP Accure in Hyderabad to touch lives globally.
FAQs
What experience is required for SP Accure Labs’ pharma jobs?
3–16 years in formulation, with mandatory hands-on experience in injectables/OSD production, QA, or QC.
Are only male candidates eligible?
Yes, this walk-in prefers male candidates only, focusing on immediate joiners with relevant pharma background.
What documents to bring to the interview?
Resume, recent increment letter, 3 months payslips/bank statements, educational certificates, Aadhar & PAN copies.
How can outstation applicants apply?
Email CV to jobs@spaccurelabs.com. Explore more at Pharma Recruiter.


