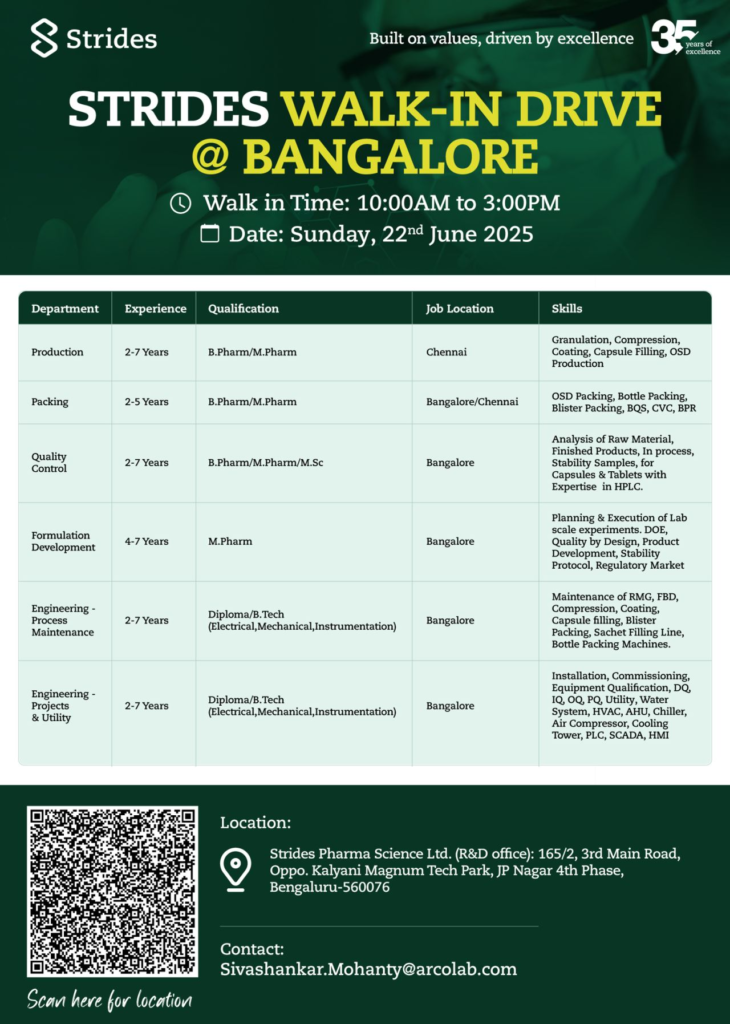
Join Strides Pharma Science Ltd, a global leader in pharmaceutical manufacturing, at our walk-in drive in Bangalore on June 22, 2025. We’re hiring for Production, Packing, Quality Control, Formulation Development, and Engineering roles to support our USFDA-approved facilities.
Contents
About Strides Pharma Science Ltd
Strides Pharma Science Ltd, headquartered in Bangalore, excels in niche finished dosage formulations, including soft gelatin capsules and tablets. With 15 manufacturing sites across six countries, we serve 100+ countries with high-quality generics. Our R&D hub drives innovation and regulatory compliance.
Job Opportunities in Bangalore and Chennai
We’re seeking candidates with 2-7 years of experience (or 4-7 years for Formulation Development) for roles in OSD manufacturing, quality control, and engineering. Candidates must have pharma experience in USFDA/MHRA-regulated plants and expertise in cGMP.
Production Department
- Experience: 2-7 Years
- Qualification: B.Pharmacy/M.Pharmacy
- Job Location: Chennai
- Skills: Expertise in granulation, compression, coating, and capsule filling.
- Responsibilities: Operate RMG, FBD, and ensure batch production with cGMP compliance.
Packing Department
- Experience: 2-5 Years
- Qualification: B.Pharmacy/M.Pharmacy
- Job Location: Bangalore/Chennai
- Skills: Proficient in blister packing, bottle packing, BQS, and CVC.
- Responsibilities: Manage packing operations and maintain batch packing records.
Quality Control Department
- Experience: 2-7 Years
- Qualification: B.Pharmacy/M.Pharmacy/M.Sc. (Chemistry)
- Job Location: Bangalore
- Skills: Analysis of raw materials, finished products, and stability samples using HPLC.
- Responsibilities: Ensure data integrity and support USFDA audits.
Formulation Development Department
- Experience: 4-7 Years
- Qualification: M.Pharmacy
- Job Location: Bangalore
- Skills: DOE, Quality by Design, and stability protocol development.
- Responsibilities: Execute lab-scale experiments and ensure regulatory compliance.
Engineering – Process Maintenance Department
- Experience: 2-7 Years
- Qualification: Diploma/B.Tech (Electrical, Mechanical, Instrumentation)
- Job Location: Bangalore
- Skills: Maintenance of RMG, compression, and blister packing machines.
- Responsibilities: Perform equipment qualification and preventive maintenance.
Engineering – Projects & Utility Department
- Experience: 2-7 Years
- Qualification: Diploma/B.Tech (Electrical, Mechanical, Instrumentation)
- Job Location: Bangalore
- Skills: Knowledge of HVAC, water systems, PLC, and SCADA.
- Responsibilities: Manage utility operations and project installations.
Interview Details
- Date: Sunday, June 22, 2025
- Time: 10:00 AM – 3:00 PM
- Venue: Strides Pharma Science Ltd. (R&D Office), 165/2, 3rd Main Road, Opp. Kalyani Magnum Tech Park, JP Nagar 4th Phase, Bengaluru-560076
- Job Locations: Bangalore/Chennai
- Requirements: Bring updated resume, educational certificates, and Aadhaar/PAN card
- Contact: Email CV to Sivashankar.Mohanty@arcolab.com
- Website: www.strides.com
Why Join Strides Pharma?
- Contribute to pharmaceuticals for 100+ countries in USFDA-approved facilities.
- Work at our R&D center driving innovative formulations.
- Enjoy a 3.9/5 rating for skill development and competitive salaries.
- Thrive in Bangalore’s pharma hub with advanced technologies.
Important Notes
- 2-7 years of pharma experience in regulated plants is mandatory.
- Candidates interviewed in the last 6 months should not apply.
- Strides does not charge recruitment fees; report suspicious activities.
- Shortlisted candidates will be contacted for further steps.
How to Prepare for the Interview
- Highlight HPLC, RMG, or QbD experience in your CV.
- Showcase cGMP compliance and audit exposure achievements.
- Bring all required documents for a seamless process.
- Prepare to discuss equipment maintenance or formulation projects.
Apply now to advance your career in pharmaceutical manufacturing. Visit www.strides.com


