Explore latest pharma jobs at Sun Pharma – Regulatory Affairs Executive in Baroda and Medical Writer in Mumbai. Ideal for M.Pharm/M.Sc & MBBS professionals seeking pharmaceutical careers in India.
Contents
About the Company
Sun Pharmaceutical Industries Ltd is one of the world’s leading generic pharmaceutical companies, committed to delivering high-quality, affordable medicines globally. With a strong focus on R&D, innovation, and regulatory excellence, Sun Pharma fosters an empowering environment.
The company encourages employees to “Create your own sunshine” through continuous growth, self-drive, and collaboration. Employees become “Better every day,” “Take charge” of their journeys, and “Thrive together” in a supportive community that values progress and impact.
Job Details
- Company Name: Sun Pharmaceutical Industries Ltd
- Experience: 1-4 years (Regulatory Affairs); 3-5 years (Medical Writer)
- Qualification: M.Sc/M.Pharm (Regulatory Affairs); MBBS (Medical Writer)
- Location: Baroda (Vadodara), Gujarat; Mumbai, Maharashtra
- Work Type: On-site
Job Description
Sun Pharma is actively hiring for two key roles in Regulatory Affairs and Clinical Research. These positions offer experienced professionals the chance to contribute to international regulatory submissions and high-quality clinical documentation in a growth-oriented, collaborative R&D environment.
Executive – Regulatory Affairs
- Department: R&D1 Regulatory Affairs
- Role: Executive (Grade G12A)
- Market: MENA (GCC, UAE, Oman, Saudi Arabia, Egypt, Iran, Iraq, Bahrain etc.)
- Experience: 1-4 years
- Education/Qualification: M.Sc / M.Pharm
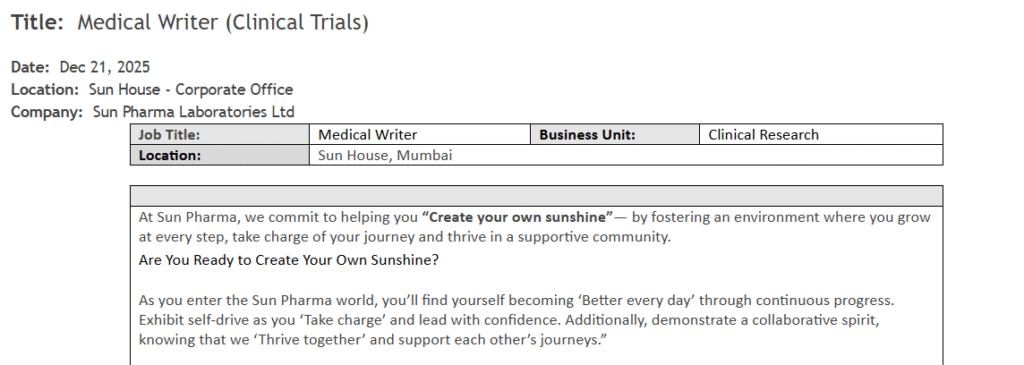
Medical Writer (Clinical Trials)
- Department: Clinical Research
- Role: Medical Writer
- Market: Clinical Trials (Interventional & Non-Interventional Studies)
- Experience: 3-5 years in CRO/Pharma/Biotech
- Education/Qualification: MBBS
Skills/Qualifications
- M.Sc/M.Pharm with 1-4 years in regulatory submissions & lifecycle management (Regulatory Affairs)
- Knowledge of CMC dossiers, deficiency responses, and country-specific variations
- MBBS with 3-5 years medical writing experience in interventional/non-interventional studies
- Proficiency in ICH-GCP, CDSCO, IJCME, GPP3, CONSORT, PRISMA, STROBE guidelines
- Formal medical writing training and publication experience in indexed journals (added advantage)
- Strong stakeholder coordination, vendor management, and quality control skills
Key Responsibilities
Executive – Regulatory Affairs:
- Review & prepare CMC dossiers for new submissions and renewals
- Review development reports, artworks, and stability protocols
- Prepare responses to regulatory deficiencies for timely approvals
- Manage lifecycle variations (API/site changes, harmonization)
- Ensure regulatory compliance, circulate approval packages, and update repositories
Medical Writer (Clinical Trials):
- Develop/update SOPs, process documents, and trackers
- Prepare/review protocols, IB, CRF, ICD, CSR, and patient diaries
- Ensure alignment with ICH-GCP, CDSCO, and relevant guidelines
- Conduct QC and lead review cycles with stakeholders
- Finalize publication plans and prepare manuscripts, abstracts, posters
- Coordinate with internal/external stakeholders and manage vendors
Benefits/Perks
- Robust benefits focused on well-being and success
- Strong emphasis on personal and professional growth
- Supportive community for continuous progress
- Opportunities to collaborate and make lasting impact
- Environment that encourages self-drive and leadership
- Career development in a globally respected pharma leader
How to Apply
Apply directly through the official Sun Pharma careers portal at https://careers.sunpharma.com/. Search for “Executive – Regulatory Affairs” (Baroda) or “Medical Writer” (Mumbai) positions and submit your updated resume.
Verified Post
Verification: To confirm the legitimacy of this posting, you can view the original announcement on the Sun Pharma Career page.

For additional pharma job opportunities across India, visit Pharma Recruiter. Take the next step in your pharmaceutical career – apply today!
Why You Should Join
At Sun Pharma, you’ll join a culture that truly invests in your success. With a focus on becoming “Better every day,” employees are empowered to take charge, lead confidently, and thrive together in a collaborative environment.
These roles offer meaningful contributions to global health, exposure to international markets, and long-term career stability in one of India’s most respected pharmaceutical companies.
FAQs
What qualifications are required for these Sun Pharma roles?
Regulatory Affairs: M.Sc/M.Pharm with 1-4 years experience. Medical Writer: MBBS with 3-5 years in medical writing for clinical studies.
How do I apply for these positions?
Visit https://careers.sunpharma.com/, search for the respective roles in Baroda or Mumbai, and apply online with your resume.
Are these roles suitable for candidates outside the specified experience range?
The roles prefer 1-4 years (Regulatory) and 3-5 years (Medical Writer), but strong matching skills may be considered.
What growth opportunities does Sun Pharma provide?
Sun Pharma offers continuous learning, leadership development, collaboration, and clear pathways for professional advancement in a supportive environment.


