Sun Pharmaceutical Industries Ltd., the world’s fourth-largest specialty generic pharmaceutical company and India’s largest pharmaceutical enterprise, is renowned for delivering high-quality medicines trusted in over 150 countries. With multiple state-of-the-art manufacturing facilities and R&D centers, we are committed to innovation and excellence.
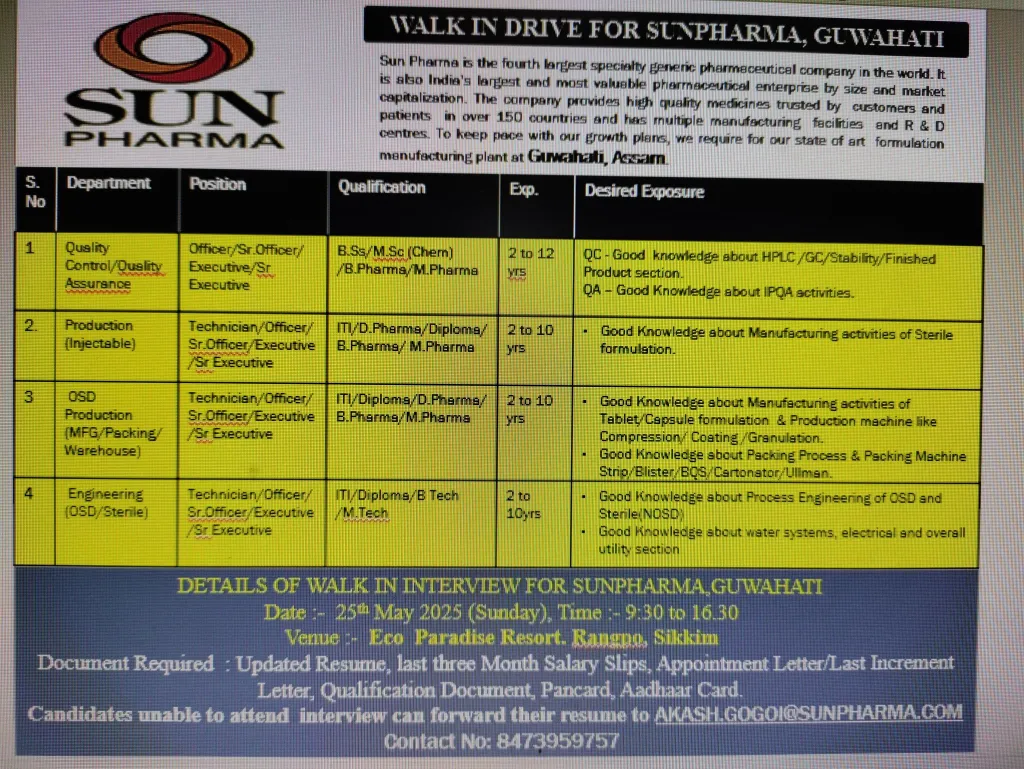
We are hosting a walk-in interview on May 25, 2025, in Rangpo, Sikkim, for roles at our advanced Guwahati, Assam formulation plant. Join our team to contribute to our mission of enhancing global healthcare!
Note: Candidates must have experience in pharmaceutical formulations (OSD or sterile injectables). API-only experience is not eligible. Sun Pharma does not charge any fees for job applications. Beware of fraudulent invitations requesting payment; contact AKASH.GOGOI@SUNPHARMA.COM for verification.
Contents
Interview Details
- Date: Sunday, May 25, 2025
- Time: 9:30 AM to 4:30 PM
- Venue: Eco Paradise Resort, Rangpo, Sikkim
- Requirements: Bring updated resume, last 3 months’ salary slips, appointment letter/last increment letter, qualification documents, PAN card, and Aadhar card.
- Unable to Attend? Email your CV to AKASH.GOGOI@SUNPHARMA.COM. Mention the position and department in the subject line (e.g., “QC Officer Application”).
- Contact: +91 8473959757
- Website: Sun Pharma
Available Positions
1. Quality Control (QC)
- Position: Officer / Sr. Officer / Executive / Sr. Executive
- Qualification: B.Sc / M.Sc (Chemistry) / B.Pharm / M.Pharm
- Experience: 2-12 Years
Desired Exposure:
- Expertise in HPLC, GC, stability studies, and finished product analysis.
- Proficiency in analytical instruments (e.g., UV, FTIR, dissolution, KF titrator) and software like Empower.
- Conduct method validation, method transfer, and stability sample analysis per ICH guidelines.
- Manage OOS, OOT, deviations, CAPA, and QMS documentation.
- Ensure compliance with cGMP, USFDA (21 CFR Part 210, 211), and WHO-GMP standards.
- Support regulatory audits and instrument calibrations (IQ, OQ, PQ).
2. Quality Assurance (QA)
- Position: Officer / Sr. Officer / Executive / Sr. Executive
- Qualification: B.Pharm / M.Pharm
- Experience: 2-12 Years
Desired Exposure:
- Perform IPQA activities, including line clearance, in-process checks, and batch record reviews.
- Handle QMS, deviations, CAPA, market complaints, and APQR preparation.
- Support process validation, cleaning validation, and regulatory audits (USFDA, WHO).
- Ensure compliance with cGMP and SOPs for OSD and sterile formulations.
3. Production (Injectable)
- Position: Technician / Officer / Sr. Officer / Executive / Sr. Executive
- Qualification: ITI / Diploma / D.Pharm / B.Pharm / M.Pharm
- Experience: 2-10 Years
Desired Exposure:
- Manage manufacturing activities for sterile formulations (e.g., ampoules, vials, PFS).
- Operate equipment like autoclaves, filling machines, and lyophilizers.
- Handle BMR/BPR documentation, media fills, and aseptic process validations.
- Ensure compliance with cGMP and sterile manufacturing standards.
4. OSD Production (Manufacturing/Packing/Warehouse)
- Position: Technician / Officer / Sr. Officer / Executive
- Qualification: ITI / Diploma / D.Pharm / B.Pharm / M.Pharm
- Experience: 2-10 Years
Desired Exposure:
- Operate tablet/capsule manufacturing equipment (e.g., FBD, RMG, compression, coating machines like Fette, Sejong).
- Manage packing processes using strip/blister/BQS/cartonator/Ullman machines.
- Handle raw material dispensing, inventory control, and PPIC activities in the warehouse.
- Ensure compliance with cGMP, SOPs, and batch documentation.
5. Engineering (OSD/Sterile)
- Position: Technician / Officer / Sr. Officer / Executive / Sr. Executive
- Qualification: ITI / Diploma / B.Tech / M.Tech
- Experience: 2-10 Years
Desired Exposure:
- Maintain and troubleshoot process engineering systems for OSD and sterile (non-OSD) facilities.
- Manage water systems, HVAC, electrical systems, and utility equipment.
- Perform equipment qualifications (DQ, IQ, OQ, PQ) and preventive maintenance.
- Ensure compliance with cGMP and safety standards during regulatory audits.
Why Join Sun Pharma?
- Global Leader: Work with the world’s fourth-largest generic pharmaceutical company, trusted in 150+ countries.
- Innovative Environment: Contribute to cutting-edge OSD and sterile formulation manufacturing in a state-of-the-art Guwahati facility.
- Career Growth: Access world-class training, mentorship, and career advancement opportunities.
- Benefits: Competitive salary, health insurance, performance incentives, and a supportive work culture (rated 3.7/5 for job security on AmbitionBox).
Learn more about our mission at Sun Pharma and industry standards at USFDA or WHO.
How to Apply
- Attend the Walk-in: Bring all required documents to Eco Paradise Resort, Rangpo, Sikkim, on May 25, 2025.
- Email Application: If unable to attend, send your CV to AKASH.GOGOI@SUNPHARMA.COM.
- Stay Updated: Follow #PharmaJobs and #GuwahatiJobs on X.com for the latest pharmaceutical career opportunities.
Join Sun Pharma to drive innovation and quality in global healthcare!


