Join Umedica Laboratories‘ USFDA, EU GMP & TGA-approved formulation plant in Vapi. Multiple production, packing, QC, injectable & engineering roles available in this walk-in interview for pharma professionals.
Contents
- 1 About the Company
- 2 Job Details
- 3 Job Description
- 3.1 Officer / Sr. Officer – Tablet Manufacturing
- 3.2 Technical Associate – Tablet Manufacturing
- 3.3 Officer / Sr. Officer – Tablet Packing
- 3.4 Technical Associate – Tablet Packing
- 3.5 Officer / Sr. Officer – Engineering & Maintenance
- 3.6 Technician – Engineering & Maintenance
- 3.7 Officer / Sr. Officer – Quality Control (Analytical)
- 3.8 Officer / Sr. Officer – Packaging Design (ITD)
- 3.9 Officer / Executive – Regulatory Affairs
- 3.10 Officer / Sr. Officer – Injectable Manufacturing
- 3.11 Operator – Injectable Manufacturing
- 4 Skills/Qualifications
- 5 Key Responsibilities
- 6 Benefits/Perks
- 7 How to Apply
- 8 Walk-in Interview Details
- 9 Why You Should Join
- 10 FAQs
About the Company
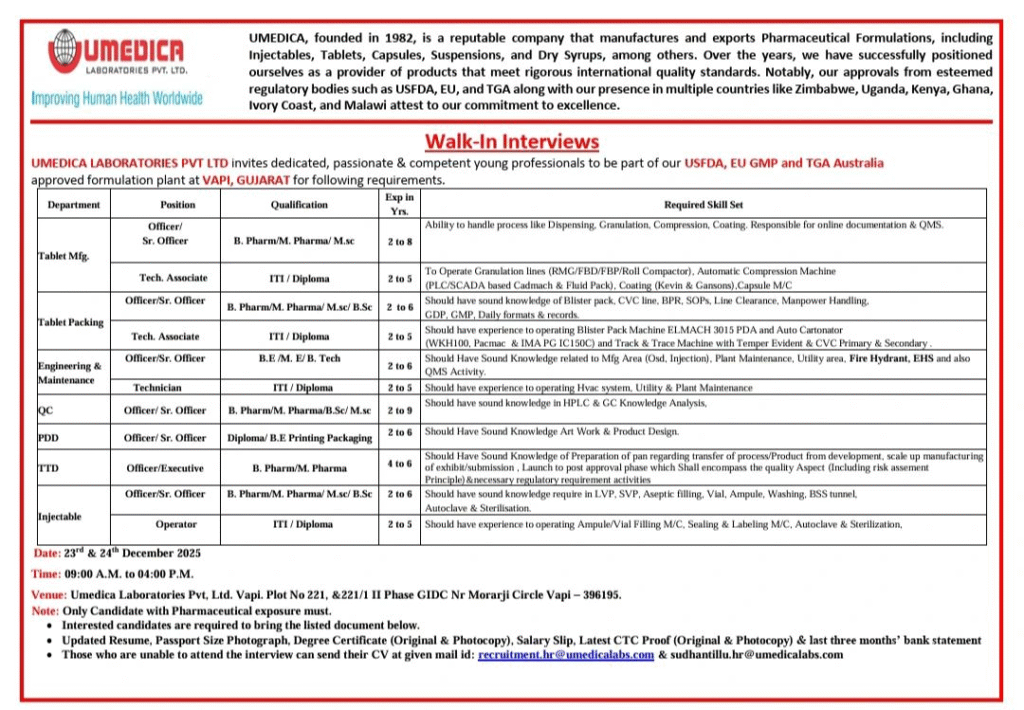
Founded in 1982, Umedica Laboratories Pvt. Ltd. is a trusted name in pharmaceutical manufacturing and exports. The company produces high-quality formulations including injectables, tablets, capsules, suspensions and dry syrups. With approvals from stringent regulatory bodies such as USFDA, EU GMP and TGA Australia, Umedica maintains exceptional quality standards.
The company has a strong global presence with successful operations in countries including Zimbabwe, Uganda, Kenya, Ghana, Ivory Coast and Malawi. Umedica continues to grow as a reliable partner in improving human health worldwide.
Job Details
- Company Name: Umedica Laboratories Pvt. Ltd.
- Experience: 2–9 years (varies by role)
- Qualification: B.Pharm, M.Pharm, M.Sc, B.Sc, B.E/B.Tech/M.E, Diploma, ITI
- Location: Vapi, Gujarat
- Work Type: On-site
Job Description
Umedica Laboratories is conducting walk-in interviews to hire dedicated and competent professionals for its state-of-the-art formulation facility in Vapi.
Openings span multiple departments including tablet manufacturing, packing, injectables, quality control, engineering, regulatory affairs and packaging design. Below are the available positions with specific requirements.
Officer / Sr. Officer – Tablet Manufacturing
- Department: Tablet Manufacturing
- Experience: 2–8 years
- Qualification: B.Pharm / M.Pharm / M.Sc
- Key Focus: Handling dispensing, granulation, compression, coating, online documentation and QMS
Technical Associate – Tablet Manufacturing
- Department: Tablet Manufacturing
- Experience: 2–5 years
- Qualification: ITI / Diploma
- Key Focus: Operating RMG, FBD, FBP, roll compactor, automatic compression and coating machines
Officer / Sr. Officer – Tablet Packing
- Department: Tablet Packing
- Experience: 2–6 years
- Qualification: B.Pharm / M.Pharm / M.Sc / B.Sc
- Key Focus: Blister packing, CVC line, BPR, SOPs, line clearance, manpower handling, GDP and GMP
Technical Associate – Tablet Packing
- Department: Tablet Packing
- Experience: 2–5 years
- Qualification: ITI / Diploma
- Key Focus: Operating blister pack machines (ELMACH 3015 PDA), auto cartonators and track & trace systems
Officer / Sr. Officer – Engineering & Maintenance
- Department: Engineering & Maintenance
- Experience: 2–6 years
- Qualification: B.E / B.Tech / M.E
- Key Focus: OSD and injection plant maintenance, utilities, fire hydrant, EHS and QMS activities
Technician – Engineering & Maintenance
- Department: Engineering & Maintenance
- Experience: 2–5 years
- Qualification: ITI / Diploma
- Key Focus: HVAC systems, utility and plant maintenance
Officer / Sr. Officer – Quality Control (Analytical)
- Department: Quality Control
- Experience: 2–9 years
- Qualification: B.Pharm / M.Pharm / B.Sc / M.Sc
- Key Focus: HPLC, GC analysis and related documentation
Officer / Sr. Officer – Packaging Design (ITD)
- Department: Packaging Design / Artwork
- Experience: 2–6 years
- Qualification: Diploma / B.E (Printing & Packaging)
- Key Focus: Artwork and product design
Officer / Executive – Regulatory Affairs
- Department: Regulatory Affairs
- Experience: 4–6 years
- Qualification: B.Pharm / M.Pharm
- Key Focus: Process transfer, scale-up, exhibit/submission batches, post-approval changes and regulatory compliance
Officer / Sr. Officer – Injectable Manufacturing
- Department: Injectable Manufacturing
- Experience: 2–6 years
- Qualification: B.Pharm / M.Pharm / M.Sc / B.Sc
- Key Focus: LVP, SVP, aseptic filling, vial/ampoule washing, tunnel sterilization and autoclave operations
Operator – Injectable Manufacturing
- Department: Injectable Manufacturing
- Experience: 2–5 years
- Qualification: ITI / Diploma
- Key Focus: Operating ampoule/vial filling, sealing, labeling machines and sterilization equipment
Skills/Qualifications
- Hands-on experience in pharmaceutical manufacturing or packing operations
- Sound knowledge of GMP, GDP, QMS and regulatory requirements
- Proficiency in operating modern machinery (RMG, FBD, compression, coating, blister packing, track & trace)
- Expertise in analytical instruments (HPLC, GC) for QC roles
- Strong documentation skills and line clearance procedures
- Ability to handle manpower, risk assessment and EHS compliance
- Relevant technical education (B.Pharm, M.Pharm, Diploma, ITI, etc.)
Key Responsibilities
- Execute manufacturing and packing processes as per SOPs
- Perform online documentation and QMS activities
- Operate and maintain advanced pharmaceutical equipment
- Ensure compliance with GMP and regulatory standards
- Conduct line clearance and manpower coordination
- Handle analytical testing and artwork design where applicable
- Support plant maintenance and utility operations
Benefits/Perks
- Opportunity to work in USFDA, EU GMP and TGA-approved facility
- Clear career growth path in a globally recognized organization
- Continuous learning and skill development
- Competitive compensation package
- Exposure to international quality standards and export markets
- Stable and supportive work culture
How to Apply
Candidates unable to attend the walk-in can email their updated resume to: recruitment.hr@umedicalabs.com and sudhantillu.hr@umedicalabs.com
You can also explore more pharma opportunities and get expert guidance at Pharma Recruiter.
Don’t miss this chance to build your pharmaceutical career with a reputed exporter—apply today!
Walk-in Interview Details
- Date: 23 & 24 December 2025
- Time: 09:00 AM to 04:00 PM
- Venue: Umedica Laboratories Pvt. Ltd., Plot No. 221 & 221/1, II Phase, GIDC, Near Morarji Circle, Vapi – 396195, Gujarat
- Documents Required: Updated resume, passport-size photograph, original & photocopy of degree certificates, latest salary slip, CTC proof and last three months’ bank statements
Note: Only candidates with pharmaceutical industry experience will be considered.
Why You Should Join
Joining Umedica means becoming part of a company committed to excellence and innovation in pharmaceutical formulations. With approvals from top global regulators and a presence across multiple continents, employees gain valuable exposure to international standards.
The organization fosters professional growth, encourages learning and offers long-term career stability in a compliant and progressive environment.
FAQs
Who is eligible to attend the walk-in interview?
Only candidates with relevant pharmaceutical industry experience and the specified qualifications are eligible.
Can freshers apply for these positions?
No, all roles require a minimum of 2 years of experience in the pharma sector.
What if I cannot attend the walk-in?
You can email your resume to recruitment.hr@umedicalabs.com and sudhantillu.hr@umedicalabs.com.
Is there any application fee?
No, the walk-in interview and application process are completely free.
What growth opportunities are available?
Umedica offers clear career progression, international exposure and continuous training in a regulatory-compliant environment.


