Proper storage conditions are critical in the pharmaceutical industry to ensure the safety, efficacy, and shelf life of medicinal products. The Indian Pharmacopoeia (IP) 1996 outlines specific standards for temperature ranges and container types to prevent drug degradation and ensure compliance with Good Manufacturing Practices (GMP).
This comprehensive guide explains the IP 1996 storage guidelines, offering insights for pharmaceutical professionals to maintain product quality and meet regulatory requirements.
Why Proper Storage Matters
Improper storage can compromise a drug’s potency, lead to chemical degradation, or even result in toxic byproducts. Adhering to IP 1996 standards is essential for pharmaceutical manufacturers, distributors, and storage facilities to ensure patient safety and pass regulatory audits.
By following these guidelines, companies can maintain product integrity, avoid costly recalls, and uphold trust in their brands.
Key Storage Conditions as per IP 1996
The IP 1996 defines specific temperature ranges and container requirements to suit various pharmaceutical products. Below is a detailed breakdown of these conditions:
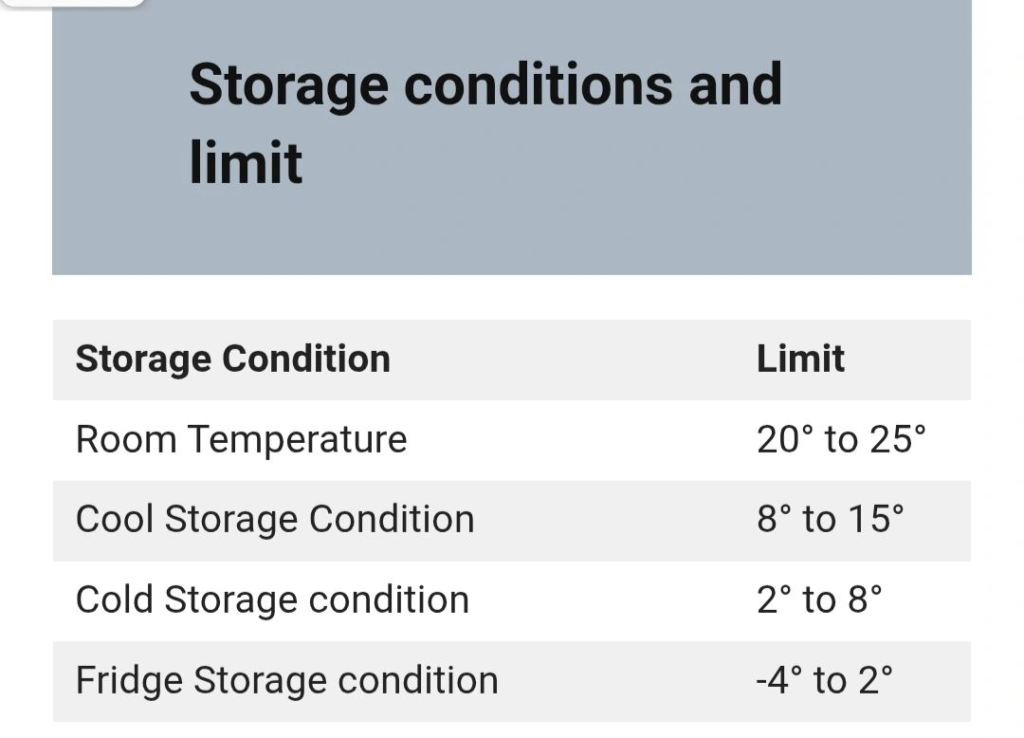
1. Cold Storage (2°C to 8°C)
Cold storage is defined as a temperature range not exceeding 8°C, typically maintained between 2°C and 8°C. This condition is critical for products like vaccines, biologics, and certain injectables that are sensitive to higher temperatures.
- Best Practice: Use a refrigerator with precise thermostat control to maintain consistent temperatures.
- Applications: Ideal for cold chain logistics and temperature-controlled storage.
- Caution: Avoid freezing unless specified, as it may damage certain formulations.
2. Cool Storage (8°C to 25°C)
Cool storage refers to a temperature range between 8°C and 25°C. Products labeled for cool storage can often be stored in a refrigerator unless otherwise specified by the manufacturer.
- Best Practice: Ensure proper ventilation and avoid temperature fluctuations.
- Applications: Suitable for many oral medications and topical products.
- Keyword Focus: Cool storage guidelines, medicine temperature stability.
3. Room Temperature
Room temperature refers to the ambient temperature of a working area, which may vary depending on geographical location and climate. This condition is typically suitable for stable formulations.
- Best Practice: Regularly monitor and record ambient temperatures to prevent unintentional overheating or cooling.
- Applications: Common for tablets, capsules, and certain syrups.
4. Warm Conditions (30°C to 40°C)
Warm conditions, ranging from 30°C to 40°C, are suitable only for specific pharmaceutical formulations designed to withstand higher temperatures.
- Caution: Not suitable for heat-sensitive drugs, as elevated temperatures may accelerate degradation.
- Applications: Limited to certain topical or robust formulations.
- Keyword Focus: Pharmaceutical climate control.
5. Excessive Heat (Above 40°C)
Temperatures above 40°C are classified as excessive heat and can cause rapid degradation of most pharmaceutical compounds.
- Warning: Avoid unless explicitly directed by the product’s storage instructions.
- Best Practice: Use temperature alarms to detect and address excessive heat exposure promptly.
Container Requirements for Pharmaceutical Storage
In addition to temperature controls, IP 1996 emphasizes the importance of proper packaging to protect drugs from environmental factors.
6. Light-Resistant Containers
Light-resistant containers, such as amber glass or opaque plastics, protect medicines from actinic (UV) light, which can degrade photosensitive compounds.
- Applications: Used for products like certain antibiotics and vitamins.
- Keyword Focus: Light-resistant pharmaceutical packaging.
7. Well-Closed Containers
Well-closed containers are designed to prevent contamination from external liquids, particles, or moisture while minimizing product loss during handling and storage.
- Applications: Common for solid dosage forms like tablets and capsules.
- Keyword Focus: GMP packaging compliance, secure drug containers.
Summary of IP 1996 Storage Conditions
| Storage Condition | Temperature Range | Key Applications | Precautions |
|---|---|---|---|
| Cold Storage | 2°C to 8°C | Vaccines, biologics | Avoid freezing unless specified |
| Cool Storage | 8°C to 25°C | Oral medications, topicals | Ensure proper ventilation |
| Room Temperature | Ambient (varies) | Tablets, capsules | Monitor for fluctuations |
| Warm Conditions | 30°C to 40°C | Specific formulations | Not for heat-sensitive drugs |
| Excessive Heat | Above 40°C | Rarely used | Avoid unless specified |
Practical Tips for Compliance
To ensure adherence to IP 1996 guidelines, pharmaceutical facilities should implement the following practices:
- Invest in Monitoring Systems: Use data loggers and temperature sensors to track storage conditions in real-time.
- Train Staff: Educate employees on proper storage protocols and the importance of GMP compliance.
- Conduct Regular Audits: Perform routine checks to verify that storage conditions meet IP 1996 standards.
- Maintain Cold Chain Logistics: Ensure seamless temperature control during transportation and storage.
The Importance of GMP Compliance
Adhering to IP 1996 storage guidelines is a cornerstone of GMP compliance. Non-compliance can lead to product recalls, regulatory penalties, and compromised patient safety. By maintaining strict control over storage conditions and using appropriate containers, pharmaceutical companies can ensure their products remain safe and effective throughout their shelf life.
Stay Informed and Compliant
Keeping up with pharmaceutical storage regulations is essential for professionals in the industry. For the latest updates on regulatory standards, GMP guidelines, and job opportunities in the pharmaceutical sector, visit PharmaRecruiter.in. Stay compliant, stay safe, and ensure the highest standards of quality in pharmaceutical storage.

