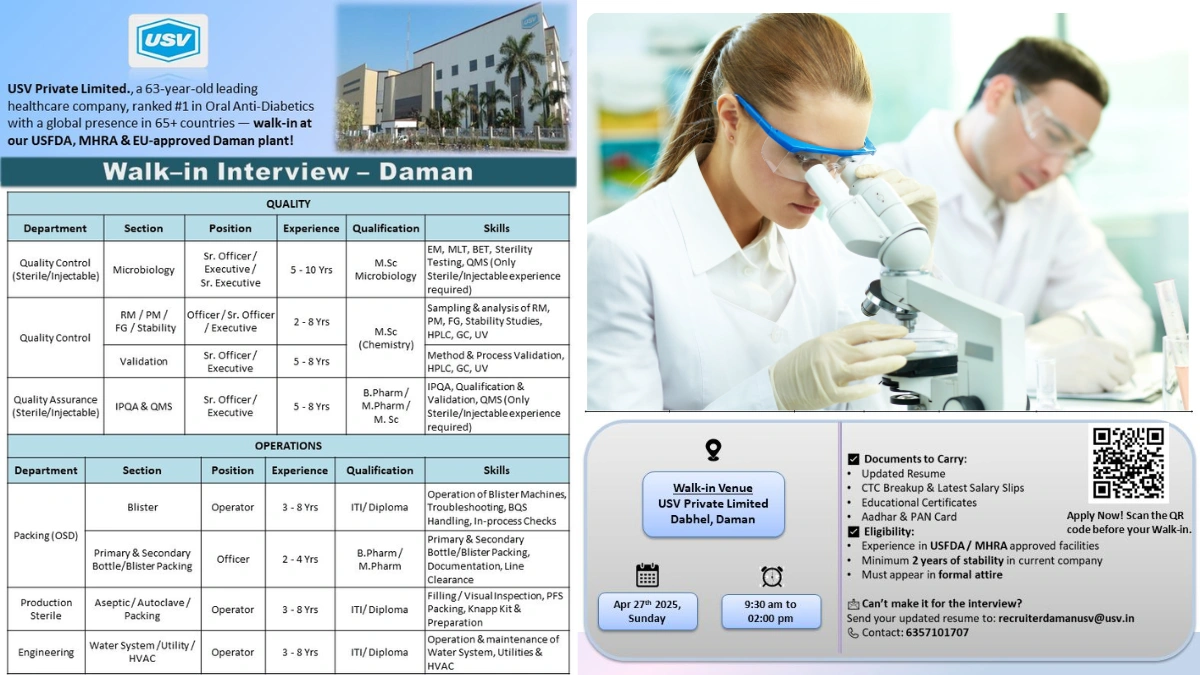
USV Private Limited, a 63-year-old Indian multinational pharmaceutical company ranked #1 in Oral Anti-Diabetics, is hosting a walk-in interview at our USFDA, MHRA, and EU-approved Daman plant. With a global presence in 65+ countries, 7 manufacturing facilities, and a workforce of 6800+, USV is a leader in cardio, diabetology, and biosimilars. Rated 4.1/5 on AmbitionBox for job security, our Daman facility offers a dynamic environment for professionals to contribute to high-quality sterile and OSD manufacturing. Join us on April 27, 2025, to be part of our mission to provide affordable healthcare worldwide.
Contents
Why Work at USV Private Limited?
USV’s Daman plant, located at Dabhel, is a cGMP-compliant facility producing sterile injectables, tablets, and capsules, with a granulation capacity of 2,150 tonnes and approvals from USFDA, MHRA, and Fimea. Despite a 2016 FDA warning letter for cGMP violations (data integrity and lab controls), USV achieved a close-out in 2018, reflecting commitment to quality. With a 3.8/5 work-life balance rating and a supportive culture, USV fosters growth and innovation.
- Work in a USFDA, MHRA, and EU-approved facility exporting to 65+ countries
- Contribute to a portfolio of 51 APIs and complex formulations like Metformin
- Benefit from a collaborative environment with 229 scientists driving R&D
- Gain exposure to advanced equipment (HPLC, GC, autoclaves) and QMS processes
Walk-In Interview Details
- Date: April 27, 2025 (Sunday)
- Time: 9:30 AM – 2:00 PM
- Venue: USV Private Limited, H-13, 16, 16A, 17, 18, 19, 20, 21 & E-22, OIDC, Mahatma Gandhi Udyog Nagar, Dabhel, Daman 396210
- Job Location: Daman, Dadra & Nagar Haveli and Daman & Diu
Requirements:
- Updated resume
- CTC breakup and latest salary slips (3 months)
- Educational certificates (mark sheets, degree certificates)
- Aadhar and PAN card copies
- Passport-size photo
Eligibility:
- Experience in USFDA/MHRA-approved facilities
- Minimum 2 years of stability in current company
- Formal attire mandatory for interview
- Candidates who attended a USV interview in the last 6 months are not eligible
Contact:
- Email resumes to recruiterdamanusv@usv.in if unable to attend
- Call 6357101707 for inquiries
- Apply by scanning the QR code in the original job posting before the walk-in
Note: USV Private Limited does not charge fees for job applications, processing, or interviews. Beware of fraudulent communications requesting payments. Verify correspondence via recruiterdamanusv@usv.in or www.usvindia.com.
Open Positions
We are hiring for Quality Control (QC), Quality Assurance (QA), Packing (OSD), Production (Sterile), and Engineering roles at our Daman plant. Candidates must have experience in sterile/injectable or OSD manufacturing and cGMP compliance in USFDA/MHRA-regulated facilities.
Quality Control (Sterile/Injectable)
Microbiology
- Position: Senior Officer / Executive / Senior Executive
- Qualification: M.Sc. (Microbiology)
- Experience: 5–10 years
Skills:
- Environmental Monitoring (EM), Microbial Limit Testing (MLT), Bacterial Endotoxin Testing (BET), and Sterility Testing
- QMS activities (deviations, CAPA) in sterile/injectable facilities
- Knowledge of cGMP and regulatory compliance
Responsibilities:
- Conduct microbial testing for products, water, and APIs
- Perform EM in cleanrooms and support sterility assurance
- Document results and manage QMS processes
RM/PM/FG/Stability
- Position: Officer / Senior Officer / Executive
- Qualification: M.Sc. (Chemistry)
- Experience: 2–8 years
Skills:
- Sampling and analysis of Raw Materials (RM), Packing Materials (PM), Finished Goods (FG), and Stability Studies
- Hands-on experience with HPLC, GC, UV-Vis Spectrophotometer
- Familiarity with Empower software and cGMP standards
Responsibilities:
- Perform chemical analysis and stability testing
- Ensure compliance with pharmacopeial standards (USP, EP)
- Support QMS and audit preparedness
Validation
- Position: Senior Officer / Executive
- Qualification: M.Sc. (Chemistry)
- Experience: 5–8 years
Skills:
- Method and Process Validation for sterile/injectable products
- Proficiency in HPLC, GC, UV, and analytical method validation (AMV)
- Knowledge of cGMP and regulatory guidelines
Responsibilities:
- Validate analytical methods and manufacturing processes
- Document validation protocols and reports
- Support regulatory inspections
Quality Assurance (Sterile/Injectable)
- Section: IPQA & QMS
- Position: Senior Officer / Executive
- Qualification: B.Pharm / M.Pharm / M.Sc.
- Experience: 5–8 years
Skills:
- In-Process Quality Assurance (IPQA) for sterile manufacturing (vials, PFS, lyophilization)
- Qualification and Validation of equipment/processes
- QMS activities (deviations, CAPA, change control) in USFDA/MHRA facilities
Responsibilities:
- Conduct IPQA checks during aseptic operations
- Manage equipment qualification and validation protocols
- Ensure cGMP compliance and audit readiness
Operations
Packing (OSD)
- Blister
- Position: Operator
- Qualification: ITI / Diploma
- Experience: 3–8 years
- Skills:
- Operation of Blister Machines (e.g., BOS, Elmach)
- Troubleshooting, BOS handling, and in-process checks
- Knowledge of cGMP and line clearance
- Responsibilities:
- Operate blister packing machines and perform quality checks
- Document packing activities and support line clearance
- Ensure compliance with SOPs
- Primary & Secondary Bottle/Blister Packing
- Position: Officer
- Qualification: B.Pharm / M.Pharm
- Experience: 2–4 years
- Skills:
- Expertise in primary and secondary bottle/blister packing
- Documentation, line clearance, and track-and-trace systems
- Familiarity with cGMP and regulatory standards
- Responsibilities:
- Supervise packing operations and ensure quality output
- Review batch records and perform line clearance
- Support regulatory audits
Production (Sterile)
- Section: Aseptic/Autoclave/Packing
- Position: Operator
- Qualification: ITI / Diploma
- Experience: 3–8 years
Skills:
- Operation of aseptic filling, autoclave, and PFS packing lines
- Visual inspection, Knapp Kit preparation, and batch manufacturing
- Knowledge of cGMP and sterile facility SOPs
Responsibilities:
- Perform aseptic operations and visual inspections
- Operate autoclaves and packing equipment
- Document processes and report deviations
Engineering
- Section: Water System/Utility/HVAC
- Position: Operator
- Qualification: ITI / Diploma
- Experience: 3–8 years
Skills:
- Operation and maintenance of water systems, utilities, and HVAC
- Troubleshooting and preventive maintenance in sterile facilities
- Familiarity with cGMP and safety protocols
Responsibilities:
- Maintain water systems, HVAC, and utilities
- Perform routine maintenance and calibrations
- Ensure compliance with regulatory standards
How to Apply
Attend the walk-in interview on April 27, 2025, from 9:30 AM to 2:00 PM at USV Private Limited, Dabhel, Daman. Arrive in formal attire by 9:30 AM for screening and bring all required documents.
Alternatively, email your updated resume to recruiterdamanusv@usv.in, specifying the position and department (e.g., “Officer QC Microbiology Application”). Apply via the QR code in the original posting for pre-registration. For inquiries, call 6357101707.
Join USV’s Mission!
Be part of a leading pharmaceutical company driving innovation in diabetes and cardio therapies. At USV Private Limited, your expertise in quality control, assurance, or operations will contribute to high-quality sterile and OSD products in our USFDA-approved Daman plant. We look forward to meeting you on April 27, 2025