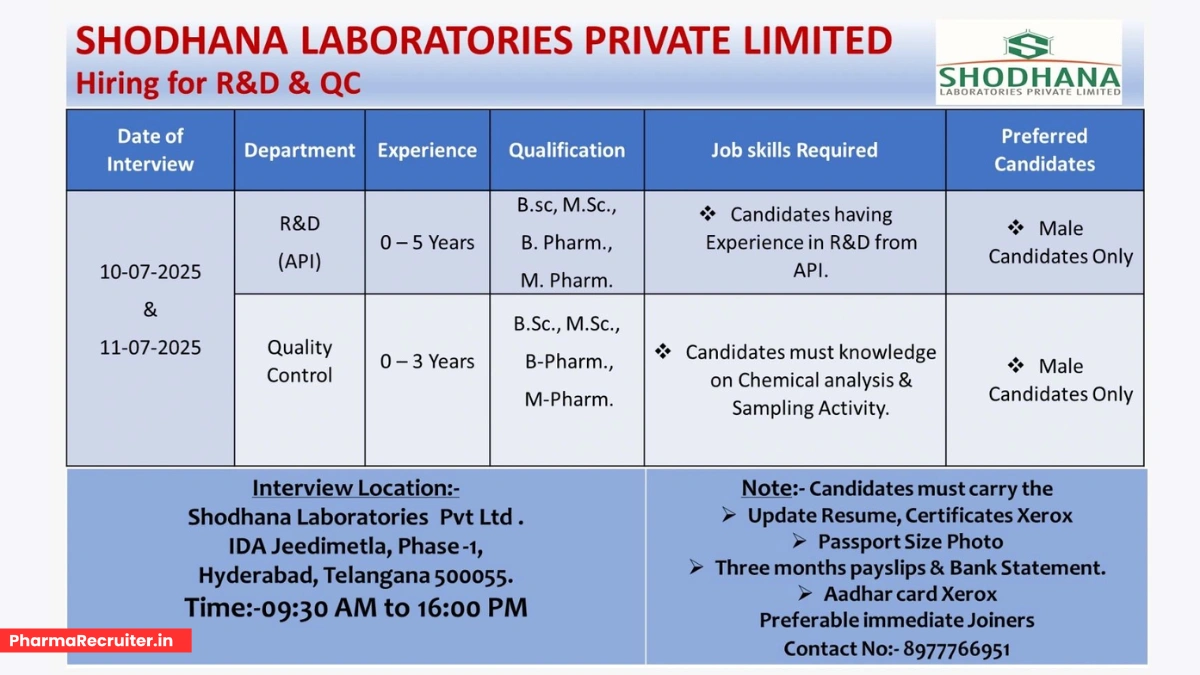
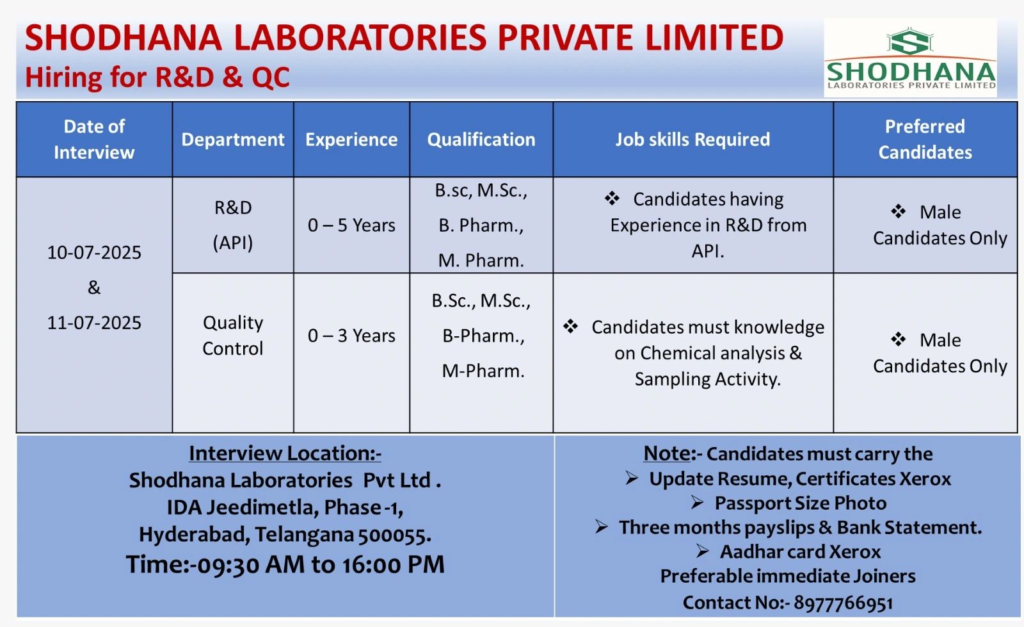
Shodhana Laboratories Private Limited, a WHO GMP, ISO 9001-2008, and ISO 14000:2004 certified pharmaceutical company, is a top-tier manufacturer of Active Pharmaceutical Ingredients (APIs) and intermediates. With advanced facilities in Hyderabad and approvals from KFDA, COFEPRIS, and TGA, we are hiring for R&D (API) and Quality Control (QC) roles. Join our dynamic team to drive innovation in healthcare!
Contents
Event Details
- Date: July 10 & 11, 2025 (Thursday & Friday)
- Time: 9:30 AM to 4:00 PM
- Venue: Shodhana Laboratories Pvt Ltd, IDA Jeedimetla, Phase-1, Hyderabad, Telangana – 500055
- Work Location: Hyderabad, Telangana
- Contact: 8977766951
- Email: Send resumes to hr@shodhana.com
Job Openings Overview
We are seeking skilled male candidates for our R&D and QC departments at our Hyderabad facility. Preference is given to immediate joiners with API experience. Below are the details:
R&D (API)
- Experience: 0-5 years
- Qualifications: B.Sc / M.Sc / B.Pharm / M.Pharm
- Job Skills:
- Experience in API research and development.
- Knowledge of chemical synthesis, process development, and GMP documentation.
- Familiarity with handling GLR, SSR, and other R&D equipment.
- Vacancies: Multiple
- Preferred Candidates: Male candidates with API R&D experience
Quality Control (QC)
- Experience: 0-3 years
- Qualifications: B.Sc / M.Sc / B.Pharm / M.Pharm
- Job Skills:
- Expertise in chemical analysis and sampling activities.
- Knowledge of cGMP, analytical techniques, and documentation.
- Familiarity with raw material, in-process, and stability sample analysis.
- Vacancies: Multiple
- Preferred Candidates: Male candidates with QC experience
Why Join Shodhana Laboratories?
Founded in 2000, Shodhana Laboratories is a fast-growing company with a world-class R&D lab and a 3.8/5 employee rating on AmbitionBox for job security. Here’s why you should join us:
- Innovative Environment: Work in a WHO GMP-certified facility with advanced technology.
- Career Growth: Opportunities for skill development despite a 3.1/5 career growth rating.
- Global Impact: Contribute to APIs supplied to leading pharmaceutical brands worldwide.
- Supportive Culture: Join a team focused on quality and customer satisfaction.
How to Prepare for the Walk-In Interview
To maximize your chances, come prepared with:
- Updated resume highlighting API R&D or QC experience.
- Copies of educational certificates (B.Sc/M.Sc/B.Pharm/M.Pharm).
- Three months’ payslips and bank statements.
- Aadhar Card, PAN Card, and passport-size photo.
- Knowledge of cGMP and regulatory requirements.
Unable to attend? Email your resume to hr@shodhana.com by July 11, 2025.
About Shodhana Laboratories
Shodhana Laboratories, headquartered in Hyderabad, specializes in API and intermediate manufacturing, adhering to ICH and cGMP guidelines. With a turnover of Rs. 100-500 crore and a team of 100+ employees, we serve global markets with high-quality products. Learn more at www.shodhana.com.
Join Shodhana Laboratories to advance your career in pharmaceutical innovation. We look forward to meeting you on July 10-11, 2025, at IDA Jeedimetla!