Zee Laboratories Limited, a leading pharmaceutical and nutraceutical company established in 1993, is hiring for multiple roles across its facilities in Karnal (Haryana), Delhi, and Paonta Sahib (Himachal Pradesh).
Known for its expertise in injectables, oral solid dosage (OSD), cosmetics, and nutraceuticals, Zee exports to 40+ countries and holds WHO-GMP, ISO, and EU-GMP certifications. Rated 3.8/5 on AmbitionBox for job security (4.0/5), Zee offers a supportive environment but faces criticism for work-life balance (3.5/5) due to rotational shifts.
Join a dynamic company with a turnover of ₹100–500 crore (FY23)!
Contents
- 1 About Zee Laboratories Limited
- 2 Open Positions
- 2.1 1. Company Secretary (CS)
- 2.2 2. International Product Manager – PMT (Pharma + Nutra + Cosmetics)
- 2.3 3. Creative Graphic Designer
- 2.4 4. Manager Production – LVP (Large Volume Parenterals)
- 2.5 5. Assistant Manager / Sr. Executive – QA (Documentation)
- 2.6 6. Manager – Quality Control (QC)
- 2.7 7. Sr. Executive – Analytical Research and Development (ARD) / Drug Regulatory Affairs (DRA)
- 3 Application Details
- 4 Why Join Zee Laboratories?
- 5 Work Locations
- 6 Important Notes
About Zee Laboratories Limited
Headquartered at 47, Industrial Area, Karnal, Haryana, Zee operates manufacturing units in Karnal and Paonta Sahib, producing large volume parenterals (LVP), tablets, capsules, ointments, and cosmetics. With 1,001–5,000 employees, Zee emphasizes quality and innovation through its DSIR-approved R&D center.
Employees praise skill development (3.9/5) but note strict SOPs and occasional management issues. Salaries range from ₹5–9L/year for senior roles, with benefits like transport and canteen.
Open Positions
Zee Laboratories is hiring for the following roles with 5–15 years of experience in pharmaceuticals, nutraceuticals, or cosmetics. Below are the details.
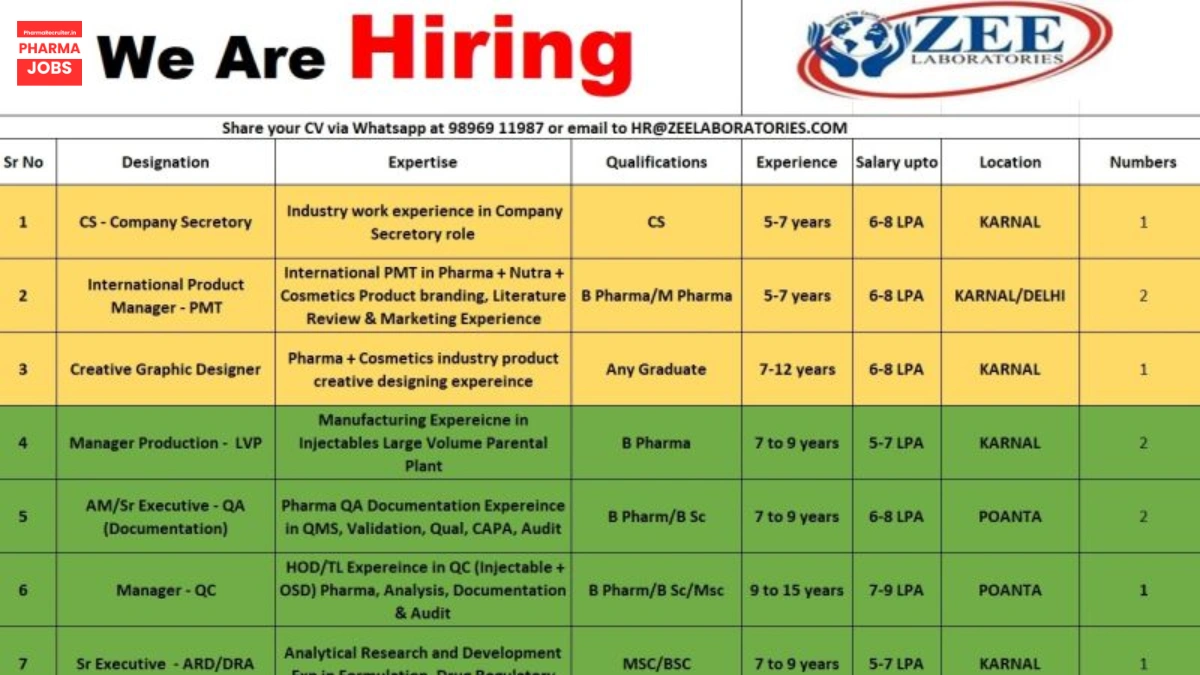
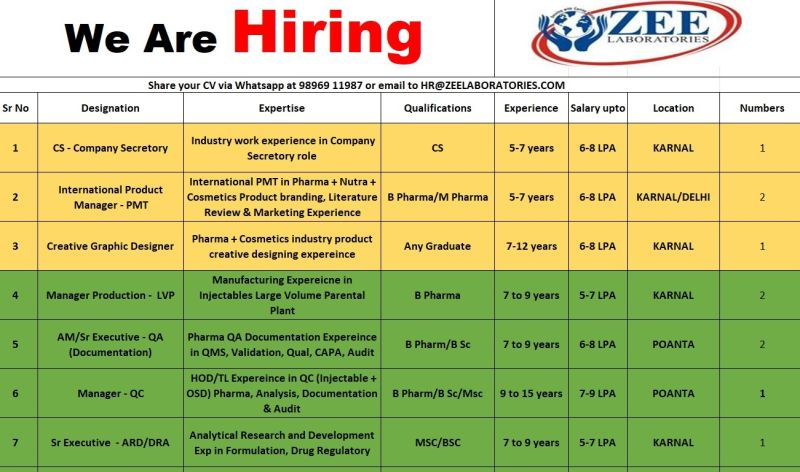
1. Company Secretary (CS)
- Qualification: CS (Company Secretary)
- Experience: 5–7 years in a Company Secretary role
- Expertise: Industry experience in corporate compliance, board meetings, and legal documentation
- Work Location: Karnal, Haryana
- Responsibilities:
- Manage corporate governance, SEBI compliance, and ROC filings
- Coordinate board meetings and maintain statutory records
- Ensure compliance with Companies Act and secretarial standards
- Liaise with auditors and regulatory authorities
- Required Skills:
- Expertise in corporate laws and compliance
- Strong documentation and communication skills
- Knowledge of SEBI, ROC, and GST regulations
- No. of Positions: 1
- Salary: Up to ₹6–8 LPA
- Note: Desk job; general shift (9 AM–6 PM).
2. International Product Manager – PMT (Pharma + Nutra + Cosmetics)
- Qualification: B.Pharm / M.Pharm
- Experience: 5–7 years in international PMT
- Expertise: Product branding, literature review, and marketing for pharma, nutraceuticals, and cosmetics
- Work Location: Karnal, Haryana / Delhi
- Responsibilities:
- Develop marketing strategies for international markets
- Conduct literature reviews and competitor analysis
- Create promotional materials and branding campaigns
- Collaborate with sales teams to achieve export targets
- Required Skills:
- Experience in international PMT for regulated markets
- Proficiency in branding and market research
- Strong presentation and analytical skills
- No. of Positions: 2
- Salary: Up to ₹6–8 LPA
- Note: General shift; travel may be required for market visits.
3. Creative Graphic Designer
- Qualification: Any Graduate
- Experience: 7–12 years in creative designing
- Expertise: Product creative designing for pharma and cosmetics industries
- Work Location: Karnal, Haryana
- Responsibilities:
- Design packaging, labels, and promotional materials
- Create visually appealing branding for tablets, injectables, and cosmetics
- Collaborate with PMT for marketing collateral
- Ensure designs comply with regulatory guidelines
- Required Skills:
- Proficiency in Adobe Photoshop, Illustrator, and CorelDRAW
- Experience in pharma/cosmetics packaging design
- Creative flair and attention to detail
- No. of Positions: 1
- Salary: Up to ₹6–8 LPA
- Note: Desk job; general shift.
4. Manager Production – LVP (Large Volume Parenterals)
- Qualification: B.Pharm
- Experience: 7–9 years in LVP injectables manufacturing
- Expertise: Manufacturing experience in large volume parenteral plants
- Work Location: Karnal, Haryana
- Responsibilities:
- Oversee LVP production (e.g., IV fluids, saline)
- Ensure WHO-GMP compliance and batch manufacturing
- Manage production schedules, labor, and equipment
- Support regulatory audits (USFDA, EU-GMP)
- Required Skills:
- Expertise in LVP manufacturing and aseptic processes
- Knowledge of GMP, QMS, and BMR/BPR
- Leadership and troubleshooting skills
- No. of Positions: 2
- Salary: Up to ₹5–7 LPA
- Note: Rotational shifts; male candidates preferred.
5. Assistant Manager / Sr. Executive – QA (Documentation)
- Qualification: B.Pharm / B.Sc
- Experience: 7–9 years in QA documentation
- Expertise: QMS, validation, qualification, CAPA, audit handling
- Work Location: Paonta Sahib, Himachal Pradesh
- Responsibilities:
- Manage QMS documentation (SOPs, protocols, reports)
- Handle CAPA, deviations, and audit compliance
- Support validation and qualification activities
- Ensure WHO-GMP and regulatory compliance
- Required Skills:
- Proficiency in QMS, CAPA, and audit preparation
- Knowledge of GMP and documentation standards
- Strong analytical and coordination skills
- No. of Positions: 2
- Salary: Up to ₹6–8 LPA
- Note: General shift with occasional flexibility.
6. Manager – Quality Control (QC)
- Qualification: M.Sc / B.Sc / B.Pharm
- Experience: 9–15 years in QC for injectables and OSD
- Expertise: HOD/Team Lead experience in analysis, documentation, and audit
- Work Location: Paonta Sahib, Himachal Pradesh
- Responsibilities:
- Lead QC team for testing injectables and OSD products
- Oversee HPLC, GC, UV, and stability analysis
- Ensure GLP compliance and audit readiness
- Manage documentation and method validation
- Required Skills:
- Expertise in HPLC, GC, and analytical testing
- Leadership in QC operations and regulatory audits
- Knowledge of GLP, GMP, and ICH guidelines
- No. of Positions: 1
- Salary: Up to ₹7–9 LPA
- Note: General shift; leadership role.
7. Sr. Executive – Analytical Research and Development (ARD) / Drug Regulatory Affairs (DRA)
- Qualification: M.Sc / B.Sc / B.Pharm
- Experience: 7–9 years in ARD or DRA
- Expertise: Analytical research, formulation development, drug regulatory affairs
- Work Location: Karnal, Haryana
- Responsibilities:
- Conduct analytical method development and validation
- Prepare dossiers for regulatory submissions
- Support formulation development for injectables/OSD
- Ensure compliance with USFDA/EU-GMP guidelines
- Required Skills:
- Proficiency in HPLC, method validation, and eCTD filings
- Knowledge of regulatory affairs and GMP
- Strong analytical and documentation skills
- No. of Positions: 1
- Salary: Up to ₹5–7 LPA
- Note: General shift; R&D-focused role.
Application Details
- How to Apply:
- Share CV via WhatsApp at +91 98969 11987
- Email CV to HR@zeelaboratories.com with subject “Application for [Position Name] – [Location] – June 2025”
- Contact: +91 98969 11987 (WhatsApp)
- Website: www.zeelaboratories.com
- Application Deadline: Not specified; apply by June 20, 2025, for priority
Documents Required:
- Updated resume with passport-sized photograph
- Educational certificates (CS/B.Pharm/M.Pharm/B.Sc/M.Sc)
- Aadhaar Card and PAN Card
- Last 3 months’ payslips and increment letter
- Experience certificates or relieving letters
Why Join Zee Laboratories?
Zee Laboratories offers a platform to work in a WHO-GMP/EU-GMP certified facility with global export exposure. Employees benefit from:
- Skill Development: Rated 3.9/5 for learning opportunities
- Global Reach: Exports to 40+ countries, including Africa and Southeast Asia
- Benefits: Transport, canteen, and competitive salaries
Innovation: DSIR-approved R&D with focus on injectables and nutraceuticals However, work-life balance (3.5/5) and management issues are concerns. Reviews note rotational shifts and strict SOPs in production/QC roles. Join a company with 30 years of pharmaceutical excellence!
Work Locations
- Karnal, Haryana: Corporate and manufacturing hub at Industrial Area, well-connected via NH-44. Ideal for desk-based (CS, PMT, ARD) and production roles.
- Delhi: PMT role with office in a commercial area (exact location unspecified). Metro connectivity and urban lifestyle.
- Paonta Sahib, Himachal Pradesh: Manufacturing unit in a pharma hub, 45 km from Dehradun. Limited public transport; personal vehicles recommended.
Important Notes
- Eligibility: Only candidates with 5–15 years of relevant pharma/nutra/cosmetics experience. Male candidates preferred for production/QC shifts.
- Shifts: CS/PMT/ARD: General shift; Production/QA/QC: Rotational shifts possible.
- Disclaimer: Zee Laboratories does not charge fees for applications. Use only official contacts +91 98969 11987 or HR@zeelaboratories.com. Beware of fraudulent offers.
- Note: Candidates interviewed in the last 6 months are not eligible. Expect technical interviews on corporate compliance, PMT strategies, LVP production, or HPLC analysis.
Don’t miss this opportunity to join Zee Laboratories Limited! Share your CV via WhatsApp at +91 98969 11987 or email HR@zeelaboratories.com by June 20, 2025, and advance your career in Karnal, Delhi, or Paonta Sahib!