Zentiva, a leading pharmaceutical company with a global presence in over 50 countries, is a producer of high-quality, affordable branded and generic medicines. With flagship manufacturing sites in Prague, Bucharest, and Ankleshwar, Zentiva employs over 4,700 people and is certified as a Great Place to Work.
We are hosting a walk-in interview on Saturday, May 24, 2025, at our Ankleshwar facility for Production, Quality Control (QC), and Quality Assurance (QA) roles. Join us to contribute to healthcare solutions that prioritize patient needs in a supportive, innovative environment!
Contents
- 1 Walk-in Interview Details
- 2 Open Positions
- 3 Candidate Requirements
- 4 How to Apply
- 5 Why Join Zentiva?
- 6 Important Notes
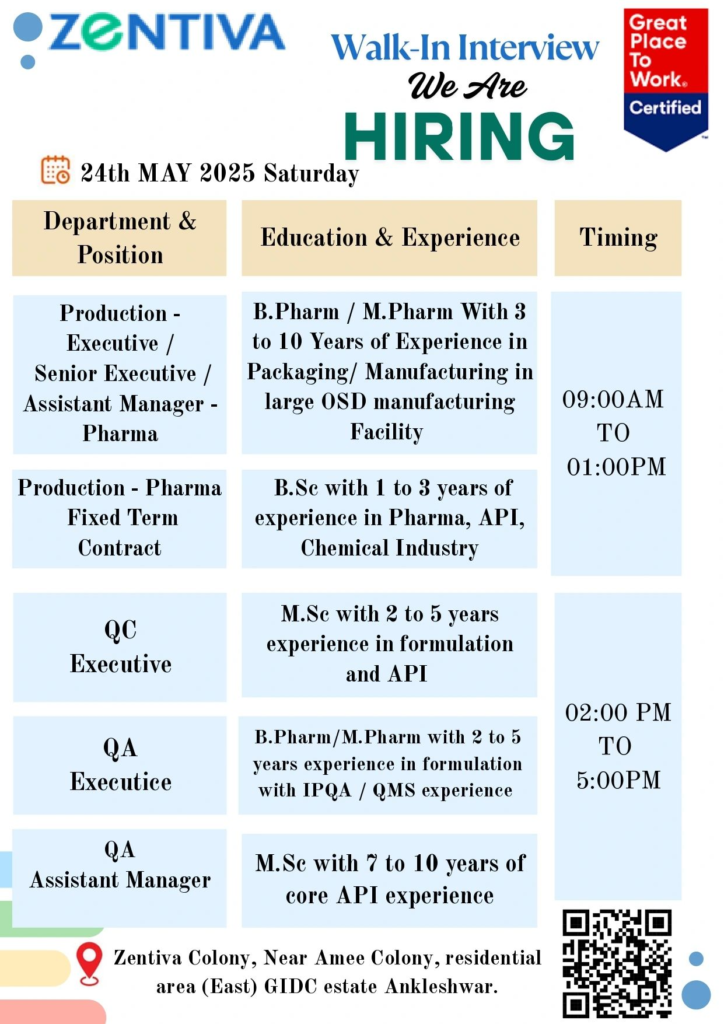
Walk-in Interview Details
- Date: Saturday, May 24, 2025
- Venue: Zentiva Colony, Near Amee Colony, Residential Area (East), GIDC Estate, Ankleshwar, Gujarat, India
- Job Location: Zentiva, Ankleshwar, Gujarat
- Application: Attend the walk-in interview with required documents
- Company Website: Zentiva
- Note: The posting is active as of May 19, 2025, 11:54 AM IST. Apply on-site during the specified timings.
Open Positions
1. Production – Executive / Senior Executive / Assistant Manager (Pharma)
Role
Oversee production operations in a large Oral Solid Dosage (OSD) manufacturing facility, focusing on packaging and manufacturing processes.
Job Specifications
- Manage granulation, compression, and coating processes in OSD manufacturing.
- Oversee packaging operations, ensuring compliance with cGMP and HSE guidelines.
- Perform SAP-related activities and ensure process validation and qualification.
Qualifications
Education
B.Pharm or M.Pharm from a reputed university.
Experience
3–10 years in OSD manufacturing, with expertise in packaging and manufacturing (e.g., granulation, compression, coating).
Skills
- Strong knowledge of cGMP, SOPs, and regulatory guidelines (e.g., USFDA, MHRA).
- Experience with SAP and serialization activities.
- Ability to optimize cycle time and ensure 100% customer service.
Interview Timing
9:00 AM
2. Production – Pharma (Fixed Term Contract)
Role
Support production activities in pharmaceutical manufacturing on a fixed-term contract basis, focusing on API or chemical processes.
Job Specifications
- Assist in API production or chemical manufacturing activities.
- Ensure adherence to cGMP, HSE, and ISO 14001 standards.
- Support daily production schedules and maintain documentation.
Qualifications
Education
B.Sc. in Chemistry or related field.
Experience
1–3 years in the pharma, API, or chemical industry.
Skills
- Basic understanding of pharmaceutical manufacturing processes.
- Familiarity with cGMP and safety protocols.
- Strong team collaboration and documentation skills.
Interview Timing
1:00 PM
3. Quality Control (QC) Executive
Role
Ensure quality standards for raw materials, intermediates, and finished products in formulation and API production.
Job Specifications
- Perform sampling and analysis of raw materials, bulk products, intermediates, and finished goods.
- Conduct stability studies, process validation, and cleaning validation sample analysis.
- Manage calibration and maintenance of lab equipment (e.g., HPLC, GC).
Qualifications
Education
M.Sc. in Organic or Analytical Chemistry.
Experience
2–5 years in formulation and API quality control within the pharmaceutical industry.
Skills
- Proficiency in HPLC, GC, and spectroscopic techniques.
- Knowledge of cGMP, ICH guidelines, and data integrity.
- Experience with out-of-specification (OOS) investigations and retesting.
Interview Timing
2:00 PM to 5:00 PM
4. Quality Assurance (QA) Executive
Role
Oversee in-process quality assurance (IPQA) and quality management systems (QMS) in formulation manufacturing.
Job Specifications
- Conduct line clearance and in-process checks on the shop floor.
- Ensure compliance with cGMP and regulatory requirements.
- Review validation reports and implement CAPA for deviations and complaints.
Qualifications
Education
B.Pharm or M.Pharm.
Experience
2–5 years in formulation manufacturing, with experience in IPQA and QMS.
Skills
- Strong understanding of cGMP, QMS, and process validation.
- Ability to perform gap analysis and drive continuous improvement.
- Excellent communication and teamwork skills.
Interview Timing
2:00 PM to 5:00 PM
5. Quality Assurance (QA) Assistant Manager
Role
Lead QA activities with a focus on API production, ensuring compliance and quality standards.
Job Specifications
- Oversee cGMP training and ensure shop floor compliance.
- Manage validation of facilities, equipment, and processes.
- Lead CAPA implementation and effectiveness reviews for API production.
Qualifications
Education
M.Sc. in Chemistry or related field.
Experience
7–10 years in core API manufacturing with QA responsibilities.
Skills
- Expertise in API quality assurance and regulatory compliance (e.g., USFDA).
- Strong leadership and problem-solving skills.
- Proficiency in documentation and audit preparation.
Interview Timing
2:00 PM to 5:00 PM
Candidate Requirements
- Documents to Bring:
- Two copies of your updated CV (highlighting relevant experience in OSD, API, QC, or QA).
- Photocopies of educational certificates (B.Pharm/M.Pharm/B.Sc./M.Sc.).
- Aadhar card, PAN card, and one passport-size photograph.
- Last 3 months’ salary slips or CTC proof (if applicable).
- Experience letters (if available).
- Application Tip: Highlight your experience with specific processes (e.g., granulation, HPLC, IPQA) and regulatory audits.
- Note: Candidates must attend during the specified timings for their role.
How to Apply
- Attend the Walk-in: Visit Zentiva Colony, Near Amee Colony, Residential Area (East), GIDC Estate, Ankleshwar, on May 24, 2025, as per the timings for your role.
- Alternative Application: Apply online at Zentiva Careers if unable to attend (based on prior Zentiva job postings).
- For Queries: Contact Zentiva’s HR team via their official website Zentiva.
Why Join Zentiva?
- Great Place to Work: Certified as a Great Place to Work, with a 4.1/5 rating for work-life balance and culture (AmbitionBox reviews).
- Global Impact: Serve patients in 50+ countries with affordable, high-quality medicines.
- Ankleshwar Facility: Work in a USFDA-compliant facility in India’s pharmaceutical hub, rated 4.1/5 for job security (AmbitionBox).
- Career Growth: Opportunities for continuous learning in a dynamic, collaborative environment, though career growth is rated at 3.1/5 (AmbitionBox).
- Employee Benefits: Salary for QA roles averages ₹3.6 Lakhs/year (AmbitionBox), with a supportive HR policy and canteen facilities.
Important Notes
- No Recruitment Fees: Zentiva does not charge fees for recruitment. Report suspicious activities via the official website.
- Eligibility: Candidates must meet the specified education and experience criteria for each role.
- Work Arrangement: This is a fully on-site role with no work-from-home options (AmbitionBox reviews).
Join Zentiva on May 24, 2025, in Ankleshwar to advance your career in pharmaceutical production, QC, and QA jobs! Bring your expertise to our facility and contribute to delivering high-quality healthcare solutions globally.


