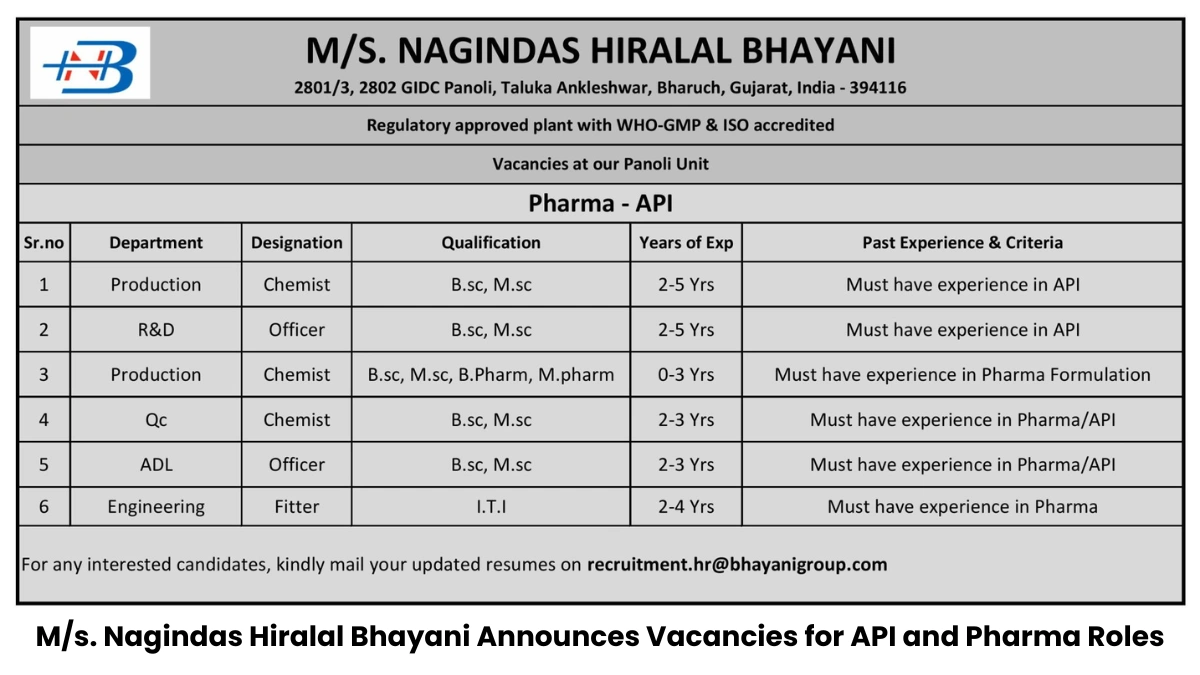
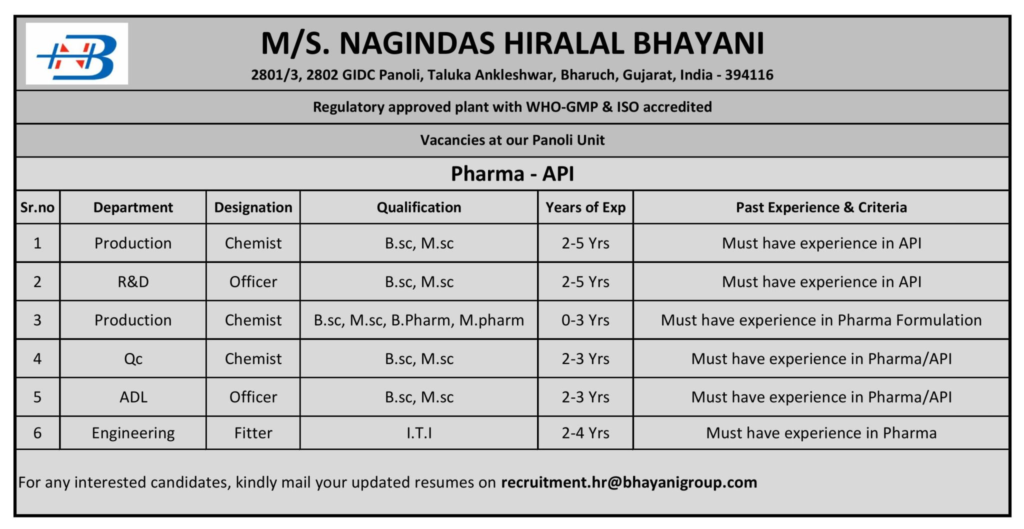
M/s. Nagindas Hiralal Bhayani, a WHO-GMP and ISO-accredited pharmaceutical manufacturing company established in 2017, is hiring for multiple roles at its state-of-the-art API and formulation plant located at Plot No. 2801/3, 2802, GIDC Panoli, Taluka Ankleshwar, Bharuch, Gujarat, India – 394116.
With a focus on external liquid preparations and disinfectants, we invite dynamic professionals to join our team in Production, R&D, Quality Control (QC), Analytical Development Laboratory (ADL), and Engineering departments as part of our recruitment drive announced on June 23, 2025.
Recruitment Details
- Application Method: Email your updated resume to recruitment.hr@bhayanigroup.com with the position title in the subject line (e.g., “Chemist – Production”)
- Job Location: M/s. Nagindas Hiralal Bhayani, 2801/3, 2802 GIDC Panoli, Taluka Ankleshwar, Bharuch, Gujarat – 394116
- Note: Only candidates with relevant pharmaceutical or API experience will be considered.
Open Positions and Requirements
Below is a detailed overview of the vacancies at our Panoli unit:
| Sr. No. | Department | Designation | Qualification | Experience | Past Experience & Criteria |
|---|---|---|---|---|---|
| 1 | Production | Chemist | B.Sc., M.Sc. | 2–5 years | Must have experience in API manufacturing |
| 2 | R&D | Officer | B.Sc., M.Sc. | 2–5 years | Must have experience in API R&D |
| 3 | Production | Chemist | B.Sc., M.Sc., B.Pharm, M.Pharm | 0–3 years | Must have experience in pharma formulation |
| 4 | Quality Control (QC) | Chemist | B.Sc., M.Sc. | 2–3 years | Must have experience in pharma/API QC |
| 5 | Analytical Development Lab (ADL) | Officer | B.Sc., M.Sc. | 2–3 years | Must have experience in pharma/API ADL |
| 6 | Engineering | Fitter | ITI (Fitter) | 2–4 years | Must have experience in pharma industry |
Responsibilities by Department
Production (Chemist – API):
- Operate and monitor API production processes (reactors, centrifugation, distillation).
- Ensure compliance with cGMP, SOPs, and batch manufacturing records (BMRs).
- Conduct in-process checks and maintain accurate documentation.
Production (Chemist – Pharma Formulation):
- Handle formulation processes for external liquid preparations (e.g., solutions, suspensions).
- Perform line clearance, in-process checks, and documentation per WHO-GMP standards.
- Support scale-up and process optimization activities.
R&D (Officer):
- Develop and optimize API synthesis routes and formulation processes.
- Conduct lab-scale experiments and support pilot plant trials.
- Document findings for regulatory submissions (e.g., DMF).
Quality Control (Chemist):
- Perform analytical testing of raw materials, intermediates, and APIs using HPLC, GC, UV, and wet chemistry.
- Ensure compliance with GLP and SOPs; support stability studies and method validation.
- Prepare data for regulatory audits (WHO-GMP, ISO).
Analytical Development Lab (Officer):
- Develop and validate analytical methods for APIs and formulations.
- Conduct impurity profiling and stability testing using advanced instrumentation.
- Support regulatory submissions with analytical data.
Engineering (Fitter):
- Perform maintenance and repair of production equipment (pumps, reactors, etc.).
- Execute preventive maintenance plans (PMP) and calibrate instruments.
- Ensure equipment uptime and compliance with safety and GMP standards.
Documents to Submit
Please include the following with your application:
- Updated resume (specify the position applied for in the subject line)
- Copies of educational certificates (B.Sc., M.Sc., B.Pharm, M.Pharm, ITI, etc.)
- Experience certificates or reference letters (mandatory to verify pharma/API experience)
- Latest passport-size photograph
- Last 3 months’ payslips and increment letter (if applicable)
- Aadhar Card and PAN Card copies
Why Join Nagindas Hiralal Bhayani?
- Regulatory Excellence: Work in a WHO-GMP and ISO-accredited facility with a drug manufacturing license (No. G/25/2262) valid until May 13, 2028, ensuring compliance with the Drugs and Cosmetics Act, 1940.
- Specialized Focus: Contribute to a modern plant designed for external liquid preparations, free from hazardous substances like beta-lactam antibiotics or cytotoxics.
- Quality Commitment: Be part of a company with a robust quality system, procuring materials from approved vendors and maintaining reserve samples per SOPs.
- Career Growth: Join a family-owned organization with a 50-year legacy, diversifying into pharmaceuticals, rigid packaging, and warehousing, offering a stable and innovative work environment.
How to Apply
Interested candidates meeting the specified experience and qualification criteria are encouraged to email their updated resume and supporting documents to recruitment.hr@bhayanigroup.com with the position title in the subject line. For inquiries, contact the HR team at +91 7572990001.
Join M/s. Nagindas Hiralal Bhayani and contribute to our mission of delivering perfect products with consistent quality, efficiency, and safety in the pharmaceutical industry! We look forward to welcoming you to our Panoli unit.